ECCO RevolutionYear: 2019
Source: Talking Heads
Authors: Renzo Cabrilli, Boris Vucelic, Miquel Gassull
Created: Friday, 28 February 2020, 3:44 PM by Dauren Ramankulov
ECCO Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel DiseaseYear: 2019
Source: JCC Issue 6, 2019
Authors: Joana Torres et al.
Created: Wednesday, 4 September 2019, 12:24 PM by Dauren Ramankulov
Patients with inflammatory bowel disease [IBD] increasingly use alternative and complementary therapies, for which appropriate evidence is often lacking. It is estimated that up to half of all patients with IBD use various forms of complementary and alternative medicine during some point in their disease course. Considering the frequent use of such therapies, it is crucial that physicians and patients are informed about their efficacy and safety in order to provide guidance and evidence-based advice. Additionally, increasing evidence suggests that some psychotherapies and mind–body interventions may be beneficial in the management of IBD, but their best use remains a matter of research. Herein, we provide a comprehensive review of some of the most commonly used complementary, alternative and psychotherapy interventions in IBD.
ECCO'22: Year: 2022
Created: Friday, 11 February 2022, 3:52 PM
ECCO-ESCP Consensus on Surgery for Crohn’s DiseaseYear: 2017
Source: JCC: Volume 12, Issue 5, 2018
Authors: Willem A. Bemelman, Janindra Warusavitarne, Gianluca M. Sampietro, Zuzana Serclova, Oded Zmora, Gaetano Luglio, Anthony de Buck van Overstraeten, John P. Burke, Christianne J. Buskens, Francesco Colombo, Jorge Amil Dias, Rami Eliakim, Tomás Elosua, I. Ethem Gecim, Sanja Kolacek, Jaroslaw Kierkus, Kaija-Leena Kolho, Jérémie H. Lefevre, Monica Millan, Yves Panis, Thomas Pinkney, Richard K. Russell, Chaya Shwaartz, Carolynne Vaizey, Nuha Yassin, André D’Hoore
Created: Friday, 31 August 2018, 10:44 AM by Dauren Ramankulov
The aim of this Consensus is to establish up-to-date standards for timing and methodology of Surgery in Crohn’s Disease.In cooperation with ESCP, ECCO Surgeons joined forces with Paediatricians as well and parts of this Guideline will cover paediatric content.
ECCO-ESGAR Consensus on Imaging techniques for assessment of IBD (2013)Year: 2013
Source: JCC: Volume 7, Issue 7, 2013
Authors: J. Panes, Y. Bouhnik, W. Reinisch, J. Stoker, S.A. Taylor , D.C. Baumgart, S. Danese, S. Halligan, B. Marincek, C. Matos, L. Peyrin-Biroulet, J. Rimola, G. Rogler, G. van Assche, S. Ardizzone, A. Ba-Ssalamah, M.A. Bali, D. Bellini, L. Biancone, F. Castiglione, R. Ehehalt, R. Grassi, T. Kucharzik, F. Maccioni, G. Maconi, F. Magro , J. Martín-Comín, G. Morana, D. Pendsé, S. Sebastian, A. Signore, D. Tolan, J.A. Tielbeek, D. Weishaupt, B. Wiarda, A. Laghi
Created: Wednesday, 29 August 2018, 5:04 PM by Dauren Ramankulov
Last Modified: Thursday, 30 August 2018, 10:45 AM by Dauren Ramankulov
The ECCO-ESGAR Consensus on Imaging techniques for assessment of IBD establishes standards for the use of cross-sectional imaging techniques in IBD. Imaging will include MRI, CT and US but not endoscopy or capsule endoscopy, even though these investigations will be at the background of all discussions.
ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspectsYear: 2018
Source: JCC: Volume 12, Issue 8
Authors: Andreas Sturm et al.
Created: Tuesday, 11 December 2018, 11:29 AM by Dauren Ramankulov
This new diagnostic consensus guideline is a joint project of the European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] that now merges the former ECCO-ESGAR Imaging Guideline and the former ECCO Endoscopy Guideline, also including laboratory parameters. It has been drafted by 30 ECCO and ESGAR members from 17 European countries.
ECCO-ESPGHAN Consensus for managing Acute Severe Ulcerative Colitis in children (2011)Year: 2011
Source: The American Journal of Gastroenterology volume 106, pages 574–588 (2011)
Authors: Dan Turner MD, PhD, Simon P L Travis FRCP, Anne M Griffiths MD, Frank M Ruemmele MD, PhD, Arie Levine MD, Eric I Benchimol MD, PhD, Marla Dubinsky MD, George Alex MBBS, FRACP, PhD, Robert N Baldassano MD, Jacob C Langer MD, Robert Shamberger MD, Jeffrey S Hyams MD, Salvatore Cucchiara MD, PhD, Athos Bousvaros MD, MPH, Johanna C Escher MD, PhD, James Markowitz MD, David C Wilson MD, Gert van Assche MD, PhD & Richard K Russell PhD
Created: Wednesday, 29 August 2018, 4:46 PM by Dauren Ramankulov
Acute severe ulcerative colitis (ASC) is a potentially life-threatening disease. We aimed to formulate guidelines for managing ASC in children based on systematic review of the literature and robust consensus process. This manuscript is a product of a joint effort of the ECCO (European Crohn's and Colitis Organization), the Pediatric Porto Inflammatory Bowel Disease (IBD) Working group of ESPGHAN (European Society of Pediatric Gastroenterology, Hepatology, and Nutrition) and ESPGHAN.
Effect of baseline disease characteristics on clinical outcomes in moderate-to-severe Ulcerative Colitis treated with upadacitinib: Results from a Phase 3 trials programmeYear: 2022
Source: ECCO'22 Virtual
Authors: Peter Higgins
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundUpadacitinib (UPA) is an oral, once-daily (QD), selective Janus kinase inhibitor that has demonstrated efficacy in the induction and maintenance treatment of Ulcerative Colitis (UC). This analysis aimed to assess the impact of baseline UC characteristics on clinical outcomes following UPA therapy.
MethodsPatients (pts) with moderately to severely active UC who had failed conventional or biological treatment were enrolled in two randomised, double-blind (DB), placebo (PBO)-controlled, Phase 3, 8-week induction studies of UPA 45 mg QD vs PBO. Pts achieving a clinical response (decrease from baseline in Adapted Mayo score ≥2 points and ≥30% from baseline, plus a decrease in rectal bleeding score [RBS] ≥1 or an absolute RBS ≤1) at Week (Wk) 8 entered a 52-week, randomised, DB, PBO-controlled, Phase 3 maintenance study, and received UPA 15 mg QD, UPA 30 mg QD, or PBO. The primary efficacy endpoint was clinical remission per Adapted Mayo score at Wk 8 (induction) and at Wk 52 (maintenance). Pre-specified (PS) and post hoc (PH) analyses were conducted by baseline disease activity (full Mayo score ≤9 or >9) (PS), extent (PS), duration (<2, 2–5, >5–10, >10 years [yrs]) (PH), and high-sensitivity C-reactive protein (hs-CRP) level (≤5 or >5 mg/L) (PH).
Results988 pts were included in the induction studies, and 451 in the maintenance study. The treatment difference between UPA 45 mg QD and PBO excluded zero for all subgroups at Wk 8 (Figure 1). Wk 8 remission rates were lower for full Mayo score >9 vs ≤9, and for CRP >5 vs ≤5 mg/dL, with minimal effects of disease extent or duration. Wk 52 maintenance of remission rates were greater with UPA 15 mg and 30 mg QD vs PBO (16–44% and 30–50% difference, respectively), and with UPA 30 vs UPA 15 mg QD. This incremental benefit of the 30 mg dose was greater in pts with severely (full Mayo score >9) vs moderately active disease (Figure 2); 15 mg and 30 mg demonstrated similar efficacy in pts with Mayo score <9. Statistically non-significant numerical advantages for UPA 30 mg vs UPA 15 mg maintenance were seen in all subgroups, with the exception of hs-CRP >5 mg/L.


ConclusionUPA 45 mg QD is an effective induction treatment for UC, regardless of baseline disease characteristics. Both 15 and 30 mg UPA doses are effective maintenance regimens, regardless of baseline disease characteristics. However, the 30 mg dose shows a trend towards increased benefit vs 15 mg in pts with Mayo score >9 and extensive disease. This analysis suggests that while UPA 15 mg may be most appropriate for patients with UC with a low inflammatory burden (per full Mayo score and extent), the 30 mg dose may be more appropriate for those with a high one.
Effect of upadacitinib (UPA) treatment on extraintestinal manifestations (EIMs) in patients with moderate-to-severe Ulcerative Colitis (UC): Results from the UPA Phase 3 programmeYear: 2022
Source: ECCO'22 Virtual
Authors: Jean-Frédéric Colombel
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundEIMs are common in patients with UC and are associated with impaired quality of life. This analysis evaluated the impact of treatment with the selective Janus kinase 1 inhibitor UPA on EIMs in patients with moderate-to-severe UC.
MethodsData were included from two induction studies (U-ACHIEVE Induction [NCT02819635] and U-ACCOMPLISH [NCT03653026]) and a maintenance study (U-ACHIEVE Maintenance). Patients with moderate-to-severe UC were randomised 2:1 to 8 weeks’ induction treatment with UPA 45 mg once daily (QD) or placebo. Patients with a clinical response to induction were re‑randomised (1:1:1) to 52 weeks’ maintenance treatment with UPA 15 mg or 30 mg QD, or placebo. The presence of EIMs (peripheral arthropathy, axial arthropathy, episcleritis, uveitis, iritis, erythema nodosum, pyoderma gangrenosum, Sweet’s syndrome, oral aphthous ulcers, primary sclerosing cholangitis, autoimmune hepatitis, venous thromboembolism, chronic obstructive pulmonary disease, bronchiectasis, nephrolithiasis and anaemia) was captured in the EIM form at the start of induction (baseline) and at every visit up to Week 52. The induction study results were pooled for analysis.
ResultsAt baseline, 25.0% and 26.5% of patients in the UPA 45 mg QD and placebo induction groups had ≥1 EIM, while 24.3%, 26.6% and 24.8% of patients randomised to maintenance treatment with UPA 15 mg QD, UPA 30 mg QD or placebo, respectively, had ≥1 EIM (Table 1). The most common EIMs at baseline were anaemia, peripheral arthropathy and axial arthropathy; all other EIMs were reported in <2% of patients (Table 1). The proportion of patients reporting resolution of any EIM, arthropathy (peripheral and axial) and anaemia at Week 8 was numerically greater with UPA 45 mg QD than placebo in the induction studies (Figure 1A). Resolution at Week 52 of any EIM in patients with ≥1 EIM at baseline was significantly increased with UPA 30 mg QD (p<0.001), and numerically greater with UPA 15 mg QD, versus placebo (Figure 1B). In patients with arthropathy (peripheral or axial) at baseline, the proportion achieving resolution of arthropathy (peripheral and axial) at Week 52 was significantly increased with UPA 30 mg QD (p=0.010), and numerically higher with UPA 15 mg QD, versus placebo (Figure 1B). The same was true of anaemia resolution in patients with anaemia at baseline (p=0.019 for UPA 30 mg QD versus placebo; Figure 1B).


ConclusionUPA treatment is effective in resolving EIMs in patients with UC. EIM symptom resolution was improved versus placebo following induction treatment with UPA 45 mg and after maintenance treatment with UPA 15 or 30 mg, with the 30 mg dose providing statistically significant improvements versus placebo.
Effectiveness and Safety of tofacitinib versus vedolizumab in Patients with Ulcerative Colitis; A Nationwide, ICC Registry studyYear: 2022
Source: ECCO'22 Virtual
Authors: Tessa Straatmijer
Created: Tuesday, 24 May 2022, 8:13 PM
Background
Clinicians face difficulty in positioning biologics and JAK inhibitors in anti-TNF refractory ulcerative colitis (UC) patients. Head-to-head trials comparing the efficacy of vedolizumab and tofacitinib in UC patients are lacking. We aimed to compare the effectiveness and safety of vedolizumab and tofacitinib in anti-TNF experienced UC patients in our prospective, nationwide registry using a propensity score weighted cohort.
Methods
UC patients who failed anti-TNF treatment (with or without thiopurine) and initiated vedolizumab or tofacitinib treatment subsequently, were identified in the observational prospective Initiative on Crohn and Colitis (ICC) Registry. We selected patients with both clinical (Simple Clinical Colitis Activity Index (SCCAI) >2) and biochemical (C-reactive protein (CRP) >5mg/L or faecal calprotectin (FC) >250 µg/g) or endoscopic disease activity (endoscopic MAYO score ≥ 1) at initiation of therapy. Patients previously treated with vedolizumab or tofacitinib were excluded. Corticosteroid-free clinical remission (SCCAI<2), biochemical remission (CRP ≤5 mg/L and/or FC ≤250 µg/g) and safety outcomes were compared after 52 weeks of treatment. Inverse propensity scores weighted comparison was used to adjust for confounding and selection bias.
Results
Overall, 83 vedolizumab and 65 tofacitinib treated patients were included (table 1). Propensity score weighted analysis showed that tofacitinib treated patients were more likely to achieve corticosteroid-free clinical remission at week 12, 24 and 52 compared to vedolizumab treated patients (OR: 5.87, 95%CI:3.55-9.70, P<0.01, OR: 2.96, 95%CI: 1.85-4.73, P<0.01 and OR 2.96, 95%CI: 1.85-4.73, P<0.01, respectively) (table 2). In addition, tofacitinib treated patients were more likely to achieve biochemical remission at week 12 and week 24, remaining only statistically borderline at week 52 (OR: 2.96, 95%CI: 1.85-4.73, P<0.01, OR: 2.96, 95%CI: 1.85-4.73, P<0.01 and OR 1.68, 95%CI: 0.99-2.86, P=0.05, respectively) (table 2). There was no difference in infection rate (OR:1.057, 95%CI: 0.60-1.86, p=0.85) or severe adverse events (OR: 0.39, 95%CI: 0.03-4.33, P=0.44). No thromboembolic events were observed. Most common reason for treatment discontinuation was loss of response (table 3).



Conclusion
In tofacitinib treated, anti-TNF experienced, UC patients, we observed that a higher proportion of patients achieved corticosteroid-free remission after 12, 24 and 52 weeks compared to vedolizumab treated patients. In addition, more tofacitinib treated patients achieved biochemical remission at week 12 and 24. There was no statistically significant difference in severe adverse events.
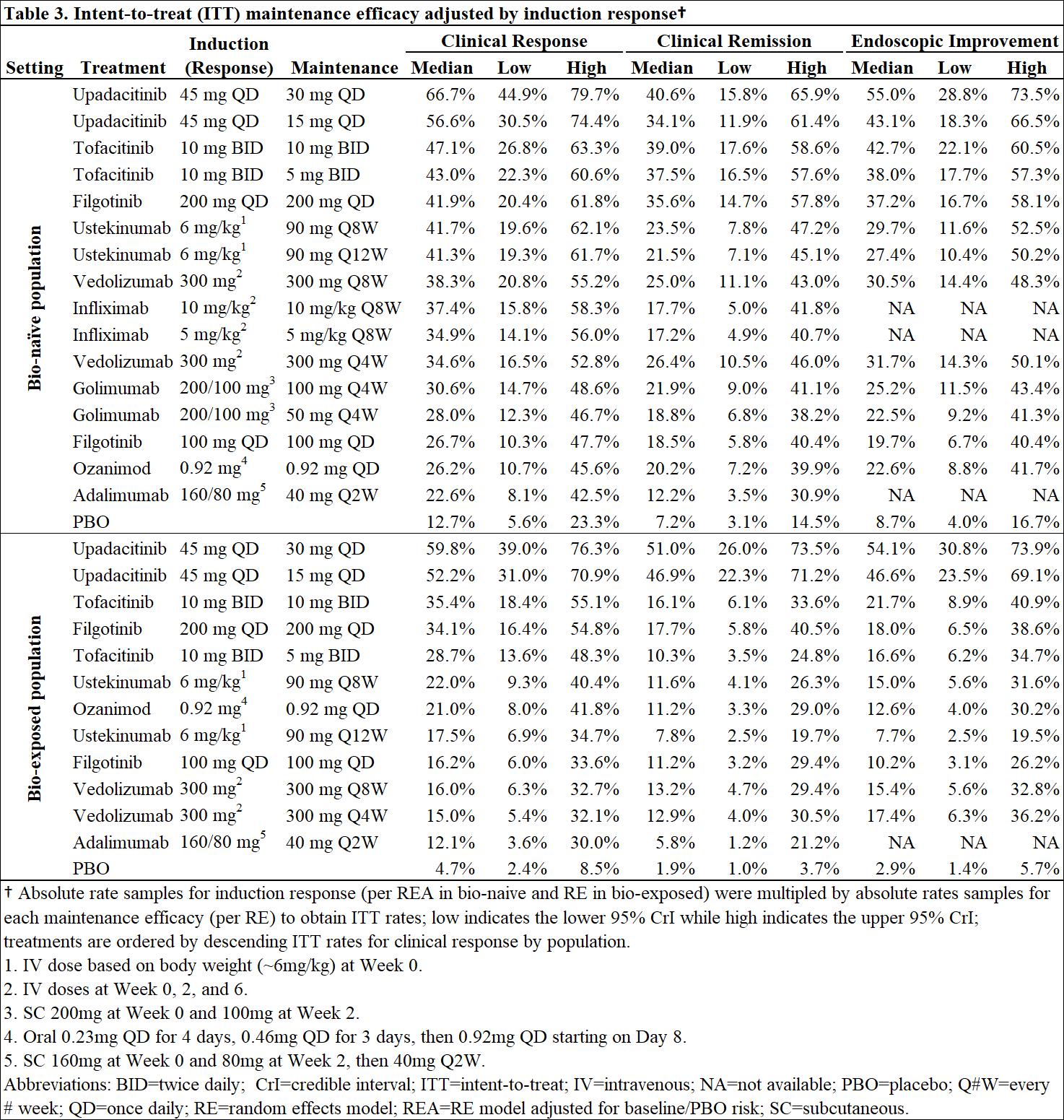
Efficacy and safety of advanced induction and maintenance therapies in patients with moderately to severely active Ulcerative Colitis: An indirect treatment comparison using Bayesian network meta-analysisYear: 2022
Source: ECCO'22 Virtual
Authors: Remo Panaccione
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundThe therapeutic armamentarium to treat adult patients with moderately to severely active ulcerative colitis (UC) continues to evolve. With this rapid innovation, the comparative efficacy and safety of more recent advanced therapies remain unknown.
MethodsBayesiannetwork meta-analysis was used to indirectly compare the efficacy and safety of advanced therapies for induction (6-10 weeks) and maintenance (44-54 weeks post-induction response) in adults with moderately-to-severely active UC. Efficacy was assessed separately in bio-naïve and bio-exposed populations by clinical remission (Full Mayo score [FM] of ≤2 with no subscore >1), clinical response (decrease from baseline in FM ≥3 points and ≥30% with decrease in rectal bleeding score [RBS] of ≥1 or absolute RBS ≤1) and endoscopic improvement (endoscopic score ≤1); ad hoc analyses were conducted on upadacitinib (UPA) RCT data to produce FM outcomes. Safety was assessed by discontinuation due to adverse events (AEs), serious AEs, and serious infections. Induction therapies included UPA 45 mg, adalimumab 160/80 mg, filgotinib 100 and 200 mg, golimumab 200/100 mg, infliximab 10 and 5 mg/kg, ozanimod 0.92 mg, tofacitinib 10 mg, ustekinumab (UST) 6 mg/kg, and vedolizumab (VED) 300 mg. The maintenance analysis included low and high maintenance doses of these therapies. Phase 3 randomized controlled trials (RCTs) were identified via systematic literature review. Random effects models were used to account for expected heterogeneity in endpoints and study design. Guidelines from the National Institute for Health and Care Excellence were followed.
ResultsOut of 31 RCTs identified, 23 were included (18 for induction and 14 for maintenance). Odds ratios vs. placebo (PBO), numbers needed to treat/harm, and surface under the cumulative ranking curve estimates are presented for efficacy in bio-naïve (Table 1) and bio-exposed (Table 2) populations and for safety in overall populations (Table 4). Intent-to-treat rates of maintenance efficacy outcomes adjusted by the likelihood of induction response show UPA to be consistently the most efficacious therapy (Table 3). There were no significant differences in serious AEs or serious infections for any advanced therapy vs PBO. For discontinuation due to AEs, only UPA had significantly lower odds vs PBO after induction, while UST and VED had significantly lower odds vs PBO after maintenance (Table 4).
ConclusionIn patients with moderately-to-severely active UC, UPA 45 mg induction and 30 mg maintenance appear more efficacious than other advanced therapies/PBO at inducing and maintaining clinical response, clinical remission, and endoscopic response, with no greater safety assessments vs PBO, over 1-year.



Efficacy and safety of bone marrow-derived mesenchymal stem cells in refractory perianal fistulae in Crohn’s Disease: Results from a prospective monocentric studyYear: 2022
Source: ECCO'22 Virtual
Authors: Catherine Reenaers
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundAnoperianal lesions affect up to 30% of patients with Crohn’s disease (CD). Long-term fistula healing is challenging with conventional biotherapies. Although recent studies demonstrated the efficacy of local injections of adipose tissue-derived stem cells with 50 % of fistulae closure without abscess at one year, this treatment is not available in routine.
The primary aim of this study was to evaluate the safety and the feasibility of the injection of bone marrow-derived mesenchymal stem cells isolated and prepared in a local university laboratory of cell therapy for perianal fistulizing CD. The second aim was to evaluate the efficacy of this treatment and his impact on the quality of life of the patients.
MethodsA prospective observational study was performed in the CHU of Liège from October 2019 till October 2021. All CD patients with perianal fistula and seton placement for at least 6 months were eligible. PRO, clinical examination, CRP, fecal calprotectine, CDAI, Short health scale (SHS) and MRI were performed at weeks 0, 12 and 48. PDAI was calculated at inclusion and at Week 48. Efficacy was defined as closure of all treated external openings at clinical examination without abscess at MRI.
ResultsSixteen patients with a median age of 49 years old and a median duration of perianal CD of 8 years were included. Eleven (69%) patients were on anti-TNF. CDAI and PDAI at inclusion were 97,5 ± 48,8 et 5 ± 4,4 respectively. Four (25%) patients reported adverse events the week after the injection (local pain 3/16, mild bleeding 1/16), all of them quickly resolutive. Ten (63%) and 8 (50%) patients had a closure of all the external opening at week 12 and 48 respectively. Five out of 6 patients with 2 external openings had at least 1 opening closed at Week 48. One abscess was observed during the follow-up. The median PDAI was numerically lower at the end of the study (3 versus 5 at the inclusion). The quality of life improved with a regression of the SHS from 10 to 7.5 at the end of the follow-up. At MRI, MAGNIFI-CD score and Van Assche index were similar for each patients at the inclusion and at the end of the study.
ConclusionInjection of locally prepared bone marrow-derived mesenchymal stem cells seems safe and effective in refractory perianal fistulae in Crohn’s disease with 50% of closure at 1 year. The treatment is associated with an improvement of the perianal activity scores and the quality of life scores but not with the MRI scores.
Efficacy and safety of combination induction therapy with guselkumab and golimumab in participants with moderately-to-severely active Ulcerative Colitis: Results through week 12 of a phase 2a randomized, double-blind, active-controlled, parallel-group, multicenter, proof-of-concept studyYear: 2022
Source: ECCO'22 Virtual
Authors: Bruce E Sands
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundPreclinical data from a murine model of acute colitis suggest that dual blockade of interleukin(IL)-23 and TNFα more effectively prevented the development of colonic inflammation than each monotherapy. Guselkumab(GUS), an IL-23p19 subunit antagonist, is being studied in inflammatory bowel disease. Golimumab(GOL), a TNFα antagonist, is approved for ulcerative colitis(UC). The objective of this study was to evaluate the efficacy and safety of combination induction therapy with GUS+GOL vs GUS or GOL alone in adults with moderately-to-severely active UC.
Methods214 patients(pts) naïve to TNFα antagonists and refractory or intolerant to conventional therapy(ie, immunomodulators and/or corticosteroids) were randomly assigned to receive GUS 200mg intravenous(IV) at weeks(wks) 0, 4, and 8(n=71); GOL 200mg subcutaneous(SC) at wk0 then 100mg SC at wks2, 6, and 10(n=72); or combination with GUS 200mg IV+GOL 200mg SC at wk0, GOL 100mg SC at wks2, 6, and 10, and GUS 200mg IV at wks4 and 8(n=71). The primary endpoint was clinical response at wk12; the major secondary endpoint was clinical remission at wk12. Other key endpoints were clinical remission based on the modified Mayo score (mMayo), symptomatic remission, endoscopic improvement, endoscopic normalization, histologic remission, composite histologic-endoscopic endpoints, and biomarker outcomes.
ResultsBaseline disease characteristics were similar among groups(Table 1), however a greater proportion of pts in both monotherapy groups had pancolitis vs the combination group. A greater proportion of pts who received combination therapy achieved clinical response at wk12(83.1%) vs GUS(74.6%) or GOL(61.1%)(Table 2). Similarly, the proportion of pts who achieved clinical remission in the combination group(36.6%) was greater than that of monotherapy groups(21.1% and 22.2%, respectively). Clinical remission by mMayo score, endoscopic improvement, histologic remission, both histologic remission and endoscopic improvement, and biomarker normalization (calprotectin, CRP) rates at wk12 were also greater in the combination group vs GUS or GOL. Percentages of pts with endoscopic normalization and both histologic remission and endoscopic normalization were nearly double with combination therapy vs either monotherapy. Adverse event(AE), serious AE, and infection rates were comparable among treatment groups. One pt receiving combination therapy experienced a serious infection of influenza and sepsis. No deaths, malignancies, or TB cases were reported through wk12.


ConclusionCombination induction treatment with GUS+GOL more effectively induced clinical response, clinical remission, and endoscopic improvement at wk12 than either monotherapy alone. AE rates were comparable among the treatment groups.
Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 inhibitor, in patients with moderately-to-severely active Ulcerative Colitis: 12-week results from the Phase 2 LATTICE-UC studyYear: 2022
Source: ECCO'22 Virtual
Authors: Silvio Danese
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundTyrosine kinase 2 (TYK2) is an intracellular kinase that mediates the signalling of key cytokines involved in ulcerative colitis (UC) pathophysiology. Deucravacitinib (DEUC) is a novel, oral, selective TYK2 inhibitor that binds to the TYK2 regulatory domain. The safety and efficacy of DEUC were evaluated in patients (pts) with moderately-to-severely active UC.
MethodsLATTICE-UC (NCT03934216), a randomised, double-blind, placebo (PBO)-controlled, multicentre, Phase 2 trial, enrolled pts with moderately-to-severely active UC (modified Mayo score of 5 to 9 [endoscopic {ES} subscore ≥2, rectal bleeding {RB} subscore ≥1, stool frequency {SF} subscore ≥2) with inadequate response, loss of response, or intolerance to ≥1 conventional or biologic therapy. Pts were randomised 2:1 to oral DEUC 6 mg or PBO twice daily (BID) and stratified by baseline (BL) corticosteroid use and prior exposure to biologics. The primary endpoint was clinical remission (modified Mayo score with subscores of SF ≤1 with ≥1-point decrease from BL, RB=0, and ES ≤1 [modified, excludes friability]) at Week (Wk) 12; endoscopic response (ES ≤1) at Wk 12 was a secondary endpoint.
ResultsDemographic and BL characteristics were generally similar across groups, except for BL disease activity as measured by the modified Mayo score and ES subscore. Most pts (63.4%) were biologic naïve, and 40.5% were receiving concomitant oral corticosteroids (Table 1). Of 131 pts randomised, 104 (79.4%) completed 12 wks of treatment (DEUC, 69/87 [79.3%]; PBO, 35/42 [83.3%]). At Wk 12, clinical remission rates were 14.8% and 16.3% in the DEUC and PBO arms, respectively, in the overall population (P=0.59); 14.0% and 25.9% in biologic-naïve pts; and 16.1% and 0.0% in biologic-experienced pts (Figure 1). At Wk 12, endoscopic response rates were 19.3% and 27.9% in the DEUC and PBO arms, respectively, in the overall population (P=0.88); 15.8% and 37.0% in biologic-naïve pts; and 25.8% and 12.5% in biologic-experienced pts (Figure 2). Pharmacodynamic data suggest insufficient inhibition of TYK2 pathways with DEUC 6 mg BID. Incidence of adverse events (AEs) was 70.1% in the DEUC arm and 47.6% with PBO; rates of serious AEs were 9.2% (n=8) and 4.8% (n=2), respectively. Rash, acne, and worsening of UC were the most common AEs in the DEUC arm. No meaningful changes from BL in mean values of laboratory parameters were observed with DEUC treatment.
ConclusionThis Phase 2 study of DEUC 6 mg BID in moderately-to-severely active UC did not meet its primary or secondary efficacy endpoints at Wk 12. In biologic-experienced pts, response rates were numerically higher with DEUC compared with PBO. The safety profile was consistent with DEUC trials in psoriasis and psoriatic arthritis.



Efficacy and safety of extended induction treatment with upadacitinib 45 mg once daily followed by maintenance upadacitinib 15 or 30 mg once daily in patients with moderately to severely active Ulcerative ColitisYear: 2022
Source: ECCO'22 Virtual
Authors: Séverine Vermeire
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundUpadacitinib (UPA), a selective and reversible Janus kinase inhibitor, has been shown to be safe and effective when administered at a dose of 45 mg once daily (QD) as 8-week induction therapy in moderate-to-severe Ulcerative Colitis (UC). This analysis evaluated outcomes following extended induction (45 mg QD for 16 weeks) followed by maintenance (15 or 30 mg QD) treatment with UPA in patients with UC who did not achieve a clinical response after 8 weeks’ induction.
MethodsPatients with moderate-to-severe UC who failed to achieve a clinical response (Adapted Mayo score decrease of ≥2 points and ≥30% from baseline, plus ≥1-point decrease in rectal bleeding score [RBS] or absolute RBS ≤1) to 8 weeks’ induction treatment with UPA 45 mg QD in the U-ACHIEVE Induction (NCT02819635) or U‑ACCOMPLISH (NCT03653026) studies, continued to receive UPA 45 mg QD in an 8‑week open-label extension. Responders at the end of the open-label extension entered the U-ACHIEVE Maintenance study and were randomised 1:1 to UPA 15 mg or 30 mg QD for 52 weeks. The efficacy endpoints were evaluated at Week 16 for the induction studies and at Week 52 for the maintenance study.
ResultsIn total, 125 patients who failed to achieve a clinical response after 8 weeks’ induction treatment received open-label UPA 45 mg for a further 8 weeks. Of these patients, 48.3% achieved a clinical response at Week 16 and were re‑randomised to UPA 15 or 30 mg in U-ACHIEVE Maintenance. Among 16-week responders who entered the maintenance study, clinical remission, maintenance of clinical response, and endoscopic improvement, respectively, at Week 52 were achieved in 33.3% versus 19.0%, 66.7% versus 35.7%, and 37.5% versus 23.8% of those who received UPA 30 versus UPA 15 mg QD as maintenance treatment (Table 1). Adverse events of special interest were reported infrequently in the two maintenance treatment groups (Table 2).


ConclusionIn this analysis, prolonged induction treatment for a total of 16 weeks was beneficial in almost half of UC patients who failed to achieve a clinical response after 8 weeks’ induction with UPA 45 mg. The benefit of maintenance therapy in these delayed responders was further demonstrated, with UPA 30 mg providing greater benefit than UPA 15 mg QD.
Efficacy and safety of filgotinib for the treatment of perianal fistulizing Crohn’s Disease: Results from the phase 2 DIVERGENCE 2 studyYear: 2022
Source: ECCO'22 Virtual
Authors: Walter Reinisch
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundTreatment of perianal fistulizing Crohn’s disease (PFCD) is a major unmet need. Filgotinib (FIL) is a once-daily, oral, preferential Janus kinase 1 inhibitor in development for the treatment of inflammatory bowel diseases. The efficacy and safety of FIL for the treatment of PFCD was evaluated in the phase 2, double-blind, randomized, placebo (PBO)-controlled DIVERGENCE 2 study (NCT03077412).
MethodsPatients (18–75 years old) with PFCD (documented diagnosis of CD for at least 3 months and 1–3 external openings [EOs] with drainage [spontaneous or on compression] for ≥ 4 weeks before screening) previously treated with antibiotics, immunomodulators and/or tumour necrosis factor inhibitors (TNFi) were randomized (2:2:1) to receive FIL 200 mg, FIL 100 mg or PBO once daily for up to 24 weeks. Active luminal CD was permitted providing that the Crohn’s Disease Activity Index score was ≤ 300 at screening. The primary endpoint was combined fistula response (reduction of ≥ 1 from baseline in the number of draining EOs determined by investigator assessment and no fluid collections > 1 cm on centrally read pelvic magnetic resonance imaging [MRI]) at Week 24. Combined fistula remission (closure of all draining EOs present at baseline and no fluid collections > 1 cm) at Week 24 was a key secondary endpoint. The study was not powered for statistical comparisons and was prematurely terminated owing to low recruitment rates during the COVID-19 pandemic.
ResultsBaseline characteristics were broadly similar across the treatment groups (Table 1). Overall, 91.2% of patients had complex perianal fistulae and TNFi treatment had previously failed in 64.9% of patients. A lower proportion of patients randomized to receive FIL 200 mg discontinued the study compared with those who received PBO (Table 2). The proportion of patients who achieved a combined fistula response at Week 24 was numerically higher in the FIL 200 mg group (47.1%; 90% confidence interval [CI]: 26.0–68.9) than in the PBO group (25.0%; 90% CI: 7.2–52.7) (Figure 1), with similar results observed for combined fistula remission (FIL 200 mg [47.1%; CI: 26.0–68.9] versus PBO [16.7%; CI: 3.0–43.8]) (Figure 2). Treatment-emergent severe adverse events were highest in the FIL 200 mg group (Table 2). Adverse event rates were otherwise similar across treatment groups.




ConclusionIn this phase 2 study, numerically higher fistula response and remission rates were observed after 24 weeks of treatment with FIL 200 mg versus PBO in patients with active PFCD and a history of multiple medical treatment failures. FIL was well tolerated overall. Further studies of FIL for the treatment of PFCD are warranted.





















