Exabis Library
Welcome to the e-CCO Library!
Efficacy and safety of filgotinib in patients with Ulcerative Colitis stratified by age: Post hoc analysis of the phase 2b/3 SELECTION and SELECTIONLTE studies
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
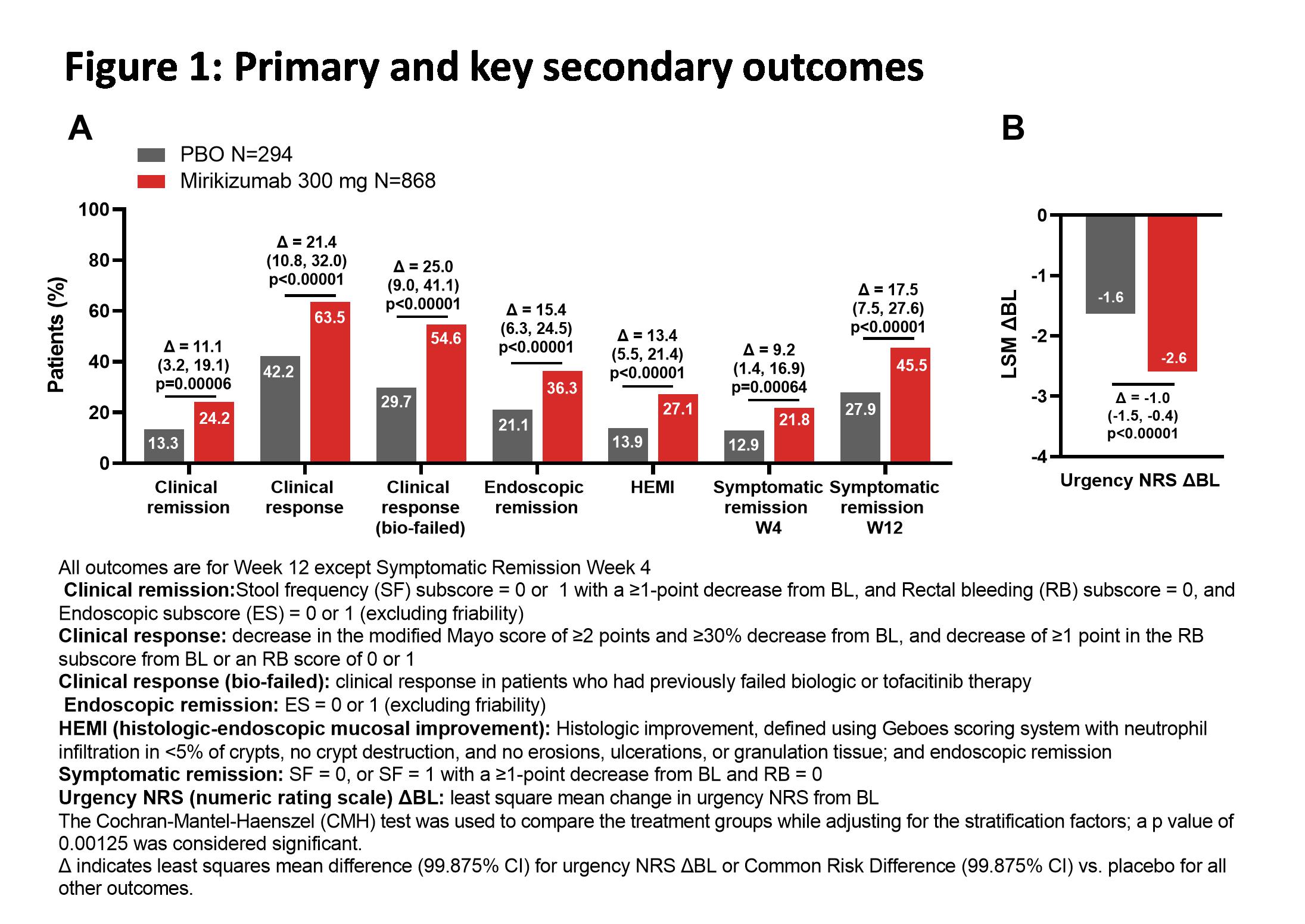
Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy and safety of vedolizumab in patients with chronic active pouchitis refractory to anti-TNF therapy: Results of a retrospective multicenter study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of biologic drugs in short-duration versus long-duration Inflammatory Bowel Disease: A systematic review and an individual-patient data meta-analysis of randomized controlled trials
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of risankizumab induction and maintenance therapy by baseline Crohn’s Disease location: Post hoc analysis of the phase 3 ADVANCE, MOTIVATE, and FORTIFY studies
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of risankizumab rescue therapy in patients with moderately to severely active Crohn’s Disease and inadequate response to risankizumab maintenance therapy
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of the treat-to-target approach in modifying disease course with ustekinumab in patients with moderate-to-severe Crohn’s Disease: Results from the STARDUST trial
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Elastography and CEUS to characterize strictures and abdominal masses in Crohn’s Disease
2019
6th ECCO-ESGAR Ultrasound Workshop - Advanced
Wednesday, 5 June 2019, 9:01 PM
Elastography and CEUS to characterize strictures and abdominal masses in Crohn’s Disease
2019
6th ECCO-ESGAR Ultrasound Workshop - Advanced
Tuesday, 28 May 2019, 3:32 PM
1
Endoscopic and imaging features of CD, intestinal TB,
2017
2nd ECCO-AOCC Forum: Learning from the Masters
1
Endoscopic Assessment and Management of Strictures
2019
Skills Videos
Friday, 22 February 2019, 2:53 PM by ECCO Administrator
Wednesday, 2 June 2021, 2:01 PM by ECCO Administrator
Endoscopic Assessment of UC Activity with UCEIS e-Course
2016
e-Course
Thursday, 27 February 2020, 4:49 PM by Dauren Ramankulov
Wednesday, 2 June 2021, 1:14 PM by ECCO Administrator
Endoscopic outcomes and therapeutic drug monitoring with Vedolizumab: the Leuven experience
2019
JCC Podcast
Wednesday, 9 October 2019, 2:04 PM by Dauren Ramankulov
Tuesday, 13 October 2020, 3:49 PM by Dauren Ramankulov
Endoscopic Scoring in IBD e-Course
2019
e-Course
Friday, 28 February 2020, 11:42 AM by Dauren Ramankulov
Friday, 13 January 2023, 11:32 AM by ECCO Administrator
Endoscopic Scoring Systems in Crohn's Diesase
2019
Skills Videos
Friday, 22 February 2019, 2:57 PM by ECCO Administrator
Wednesday, 2 June 2021, 3:40 PM by ECCO Administrator
Endoscopic scoring systems in Crohn’s disease
2019
Skills Video
Friday, 28 February 2020, 3:52 PM by Dauren Ramankulov
Monday, 17 August 2020, 10:59 AM by Dauren Ramankulov
Endoscopy and IBD incl. chromo-endoscopy, balloon dilatation
2019
17th IBD Intensive Advanced Course
Tuesday, 28 May 2019, 3:32 PM
1