European Crohn’s and Colitis Organisation Topical Review on IBD in the ElderlyYear: 2017
Source: JCC: Volume 11, Issue 3, 2017
Authors: Andreas Sturm, Christian Maaser, Michael Mendall, Dimitrios Karagiannis, Pantelis Karatzas, Nienke Ipenburg, Shaji Sebastian, Fernando Rizzello, Jimmy Limdi, Konstantinos Katsanos, Carsten Schmidt, Steven Jeuring, Francesco Colombo, Paolo Gionchetti
Created: Friday, 31 August 2018, 11:35 AM by Dauren Ramankulov
Last Modified: Friday, 22 February 2019, 10:39 AM by ECCO Administrator
This ECCO topical review of the European Crohn’s and Colitis Organisation [ECCO] focuses on the epidemiology, pathophysiology, diagnosis, management and outcome of the two most common forms of inflammatory bowel disease, Crohn’s disease and ulcerative colitis, in elderly patients. The objective was to reach expert consensus to provide evidence-based guidance for clinical practice.
European Crohn’s and Colitis Organisation Topical Review on Prediction, Diagnosis and Management of Fibrostenosing Crohn’s DiseaseYear: 2016
Source: JCC: Volume 10, Issue 8, 2016
Authors: Florian Rieder, Giovanni Latella, Fernando Magro, Elif S. Yuksel, Peter D. R. Higgins, Antonio Di Sabatino, Jessica R. de Bruyn, Jordi Rimola, Jorge Brito, Dominik Bettenworth, Gert van Assche, Willem Bemelman, Andre d’Hoore, Gianluca Pellino, Axel U. Dignass
Created: Friday, 31 August 2018, 11:31 AM by Dauren Ramankulov
This ECCO topical review of the European Crohn’s and Colitis Organisation [ECCO] focused on
prediction, diagnosis, and management of fibrostenosing Crohn’s disease [CD]. The objective was
to achieve evidence-supported, expert consensus that provides guidance for clinical practice
European Crohn’s and Colitis Organisation Topical Review on Transitional Care in Inflammatory Bowel DiseaseYear: 2017
Source: JCC: Volume 11, Issue 9, 2017
Authors: Patrick F. van Rheenen, Marina Aloi, Irit Avni Biron, Katrine Carlsen, Rachel Cooney, Salvatore Cucchiara, Garret Cullen, Johanna C. Escher, Jaroslaw Kierkus, James O. Lindsay, Eleftheria Roma, Richard K. Russell, Joanna Sieczkowska-Golub, Marcus Harbordl
Created: Friday, 31 August 2018, 11:54 AM by Dauren Ramankulov
Last Modified: Friday, 22 February 2019, 10:35 AM by ECCO Administrator
Background: This European Crohn’s and Colitis Organisation [ECCO] topical review focuses on the transition of adolescents with inflammatory bowel disease [IBD] from child-centred to adult-oriented care. The aim was to provide evidence-supported, expert consensus for health professionals taking part in the transition.
Methods: An online survey determined the areas of importance for health professionals involved in the transition of adolescents with IBD. Thereafter an expert panel of nine paediatric and five adult gastroenterologists was formed to identify the critical elements of the transition programme, and to prepare core messages defined as ‘current practice points’. There is limited literature about transition, therefore this review is mainly based on expert opinion and consensus, rather than on specific evidence.
Results: A total of 21 practice points were generated before the first [online] voting round. Practice points that reached >80% agreement were accepted, while those that did not reach 80% agreement were refined during a consensus meeting and subjected to voting. Ultimately, 14 practice points were retained by this review.
Conclusion: We present a consensus-based framework for transitional care in IBD that provides a guidance for clinical practice.
European Crohn’s and Colitis Organisation Topical Review on Treatment Withdrawal [‘Exit Strategies’] in Inflammatory Bowel DiseaseSource: JCC: Volume 12, Issue 1, 2017
Authors: Glen Doherty, Konstantinos H Katsanos, Johan Burisch, Matthieu Allez, Konstantinos Papamichael, Andreas Stallmach, Ren Mao, Ingrid Prytz Berset, Javier P Gisbert, Shaji Sebastian, Jarosław Kierkuś, Loris Lopetuso, Edyta Szymanska, Edouard Louis
Created: Friday, 22 February 2019, 10:26 AM by ECCO Administrator
Clinically effective therapies now exist for remission maintenance in both ulcerative colitis [UC] and Crohn’s Disease [CD]. For each major class of IBD medications [5-aminosalicyclates, immunomodulators, and biologic agents], used alone or in combination, there is a risk of relapse following reduction or cessation of treatment. A consensus expert panel convened by the European Crohn’s and Colitis Organisation [ECCO] reviewed the published literature and agreed a series of consensus practice points. The objective of the expert consensus is to provide evidence-based guidance for clinical practice so that physicians can make informed decisions in partnership with their patients. The likelihood of relapse with stopping each class of IBD medication is reviewed. Factors associated with an altered risk of relapse with withdrawal are evaluated, and strategies to monitor and allow early identification of relapse are considered. In general, patients in clinical, biochemical, and endoscopic remission are more likely to remain well when treatments are stopped. Reintroduction of the same treatment is usually, but not always, successful. The decision to stop a treatment needs to be individualized, and shared decision making with the patient should take place.
European evidence based consensus on surgery for ulcerative colitisYear: 2014
Source: JCC: Volume 9, Issue 1, 2015
Authors: Tom Øresland , Willem A. Bemelman, Gianluca M. Sampietro, Antonino Spinelli, Alastair Windsor, Marc Ferrante, Philippe Marteau, Oded Zmora, Paulo Gustavo Kotze, Eloy Espin-Basany, Emmanuel Tiret, Giuseppe Sica, Yves Panis, Arne E. Faerden, Livia Biancone, Imerio Angriman, Zuzana Serclova, Anthony de Buck van Overstraeten, Paolo Gionchetti, Laurents Stassen, Janindra Warusavitarne, Michel Adamina, Axel Dignass, Rami Eliakim, Fernando Magro, André D’Hoore
Created: Friday, 31 August 2018, 9:28 AM by Dauren Ramankulov
Last Modified: Wednesday, 23 January 2019, 5:03 PM by ECCO Administrator
The goal of this consensus initiated by the European Crohn's and Colitis Organisation (ECCO) was to establish European consensus guidelines for the surgical treatment of ulcerative colitis. The strategy to reach the consensus involved several steps and follows the standard operating procedures for consensus guidelines of ECCO. An open call for chairs and participants for this consensus was made (see acknowledgements and www.ecco-ibd). Participants were selected by the Guidelines' Committee of ECCO (GuiCom) on the basis of their publication record and a personal statement. Four working groups (WGs) were formed: WG 1 on the preoperative phase, WG 2 on the intraoperative phase, WG 3 on the postoperative phase and WG 4 on special situations. Participants were asked to answer relevant questions on current practice and areas of controversy related to the surgical treatment of ulcerative colitis based on their experience as well as evidence from the literature (Delphi procedure).
European Evidence-based Consensus: Inflammatory Bowel Disease and MalignanciesYear: 2015
Source: JCC: Volume 9, Issue 11, 2015
Authors: Vito Annese, Laurent Beaugerie, Laurence Egan, Livia Biancone, Claus Bolling, Christian Brandts, Daan Dierickx, Reinhard Dummer, Gionata Fiorino, Jean Marc Gornet, Peter Higgins, Konstantinos H. Katsanos, Loes Nissen, Gianluca Pellino, Gerhard Rogler, Franco Scaldaferri, Edyta Szymanska, Rami Eliakim
Created: Friday, 31 August 2018, 10:02 AM by Dauren Ramankulov
Due to the important clinical problem that the risk of malignancy in patients with IBD represents for physicians treating IBD, ECCO planned to initiate a programme to develop a specific Guideline for malignancy.
The aim of this consensus is to establish Guidelines for managing the risk of malignancy, treatment in the event of malignancy and therapy of IBD in the context of a past or current history of malignancy.
Evaluating segmental healing with the modified Mayo endoscopic score (MMES) has a clear additional value in predicting long-term outcome in patients with Ulcerative Colitis: Results from a prospective cohort studyYear: 2022
Source: ECCO'22 Virtual
Authors: Matthias Lenfant
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundMost endoscopic scoring systems (including the Mayo endoscopic subscore, MES) do not consider the extent of inflammation and can therefore not pick up segmental endoscopic improvement. The modified Mayo endoscopic score (MMES) was developed to overcome this limitation. We examined the predictive value of the MMES in addition to the MES on long-term clinical outcome in ulcerative colitis (UC).
MethodsBetween 2014 and 2017, patients initiating biologic therapy for active UC (baseline MES ≥2) were recruited. Patients without assessment of the upper margin of inflammation or with a clinical follow-up <12 months were excluded. We conducted a clinical and endoscopic assessment at baseline and week 8 (adalimumab, ADM) or 14 (golimumab, GOL; infliximab, IFX; vedolizumab, VDZ). Clinical response was defined as a decrease in the adapted Mayo score (excluding physician global assessment) with ≥2 points and ≥30%, plus a decrease in rectal bleeding score ≥1 or an absolute rectal bleeding score ≤1. We classified patients by evolution of endoscopic activity at week 8/14 in group A (endoscopic healing, MES ≤1), group B (segmental healing, MES >1 but decrease in MMES ≥30%) and group C (MES >1 and no drop in MMES ≥30%). Clinical relapse-free, discontinuation-free and colectomy-free survival was estimated by Kaplan-Meier analysis with log-rank test.
ResultsA total of 150 UC patients were included (52% male, median age 42 years, median disease duration 7 years) with a median (IQR) follow-up of 61 (48-68) months. An anti-TNF was initiated in 74 (35 IFX, 23 ADM, 16 GOL), and VDZ in 76 patients. A significant reduction in MES and MMES was observed at week 8/14 (cf. table). In group A 67/69 (97%) patients achieved clinical response compared to 15/27 (55%) in group B and 11/54 (20%) in group C. During follow-up 60/93 (65%) patients maintained clinical response, 83/150 (55%) patients discontinued treatment due to primary non-response or loss of response and 33/150 (22%) patients underwent colectomy. The ΔMMES demonstrated to be of additional predictive value: there was a significant difference between groups B and C regarding clinical relapse, drug persistence and need for colectomy (cf. figure).
ConclusionAlthough MES ≤1 remains the best predictor of long-term outcome, the MMES - identifying a subgroup with a segmental endoscopic response - demonstrated a clear additional value predicting long-term outcome in UC. These promising results merit inclusion of the MMES in future clinical trials.
Table
.png)
The MMES is calculated by multiplying the Mayo endoscopic subscore for the different colon segments with the maximal extent of inflammation (in decimeters) divided by the number of segments with active inflammation (Lobatón T, et al. JCC 2015; 9:846-52.)
Figure
.png)
Everything you need to know about surgeryYear: 2022
Source: 16th N-ECCO Network Meeting
Authors: Michel Adamina
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentThis presentation will provide an overview of current surgical management of IBD with a focus on abdominal and perianal manifestations and treatment of colitis ulcerosa and Crohn's disease, capitalizing on the most recent ECCO guidelines.
Evolving Role of IBD Surgery Year: 2018
Source: JCC: Volume 12, Issue 8, 2018
Authors: W A Bemelman, S-ECCO collaborators
Created: Friday, 22 February 2019, 11:04 AM by ECCO Administrator
Last Modified: Friday, 22 February 2019, 11:05 AM by ECCO Administrator
Management of patients with inflammatory bowel disease [IBD] has become increasingly complex, but at the same time very exciting and challenging. For a long time, surgery in IBD has been considered as a last resort, and although ‘multidisciplinary treatment’ has always been a popular term, involving a surgeon in daily practice was frequently limited to therapy-refractory patients. The most exciting change over the last few decades is probably the fact that involving a surgeon at an early stage of the disease is now considered good clinical practice, and is part of most quality-control parameters.
Exit StrategiesYear: 2017
Source: ECCO e-Learning
Authors: Johan Burisch, Ren Mao, Edyta Szymanska, Glen Doherty
Created: Friday, 28 February 2020, 11:04 AM by Dauren Ramankulov
Last Modified: Wednesday, 2 June 2021, 1:35 PM by ECCO Administrator
This course has been developed for gastroenterologists, surgeons, paediatricians, pathologists and other interdisciplinary medical experts interested in Inflammatory Bowel Disease(s) (IBD). One major aim of this e-learning activity is to provide evidence-based guidance for clinical practice so that physicians can make informed decisions in partnership with their patients regarding their optimal exit strategies.
After this course you will:
- To recognise risks, benefits and timing of stopping anti-TNF used as monotherapy or in combination with IM in IBD.
- To know optimal monitoring following withdrawal of biologic therapy
Exit strategyYear: 2022
Source: 20th IBD Intensive Course for Trainees
Authors: Joana Tinoco da Silva Torres; Marc Ferrante
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentLearning Objectives:
1. Indications for exit strategy
2. Management of exit strategies with biologics
3. Similar option with other drugs
Expanded genome-wide association study of Inflammatory Bowel Disease identifies 174 novel loci and directly implicates new genes in disease susceptibilityYear: 2022
Source: ECCO'22 Virtual
Authors: Laura Fachal
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundGenome-wide association studies (GWASs) have identified 243 loci associated with inflammatory bowel disease (IBD). However, the mapping of additional disease loci and causal variants is still limited by sample size. Larger GWAS can provide further insights into causal biology.
MethodsWe performed a GWAS meta-analysis of 33 cohorts, totalling 54,439 IBD patients (N=30,574 with Crohn’s disease (CD), 21,193 with Ulcerative Colitis (UC)) and 37,054 European controls. Genotype imputation was undertaken using the TOPMed diverse population panel and association tests were performed using REGENIE. These results were meta-analysed with summary statistics from 4 additional studies, a Danish cohort, the UK Biobank, deCODE, and FinnGen, totalling 73,030 IBD patients and 1 million controls, Fig1.
ResultsWe identified 174 novel genome-wide significant signals (32 associated with CD, 36 with UC, 106 with IBD, Fig2). 
Of these, 79 are located >1Mb from any known GWAS loci. We also identified two new population specific genetic associations, Fig3.

Six new loci contain genes implicated in monogenic syndromes that include colitis: CARMIL2, DOCK8, G6PC3, HPS4, NCF1, PIK3CD. Several new loci alter the expression of nearby genes, suggesting that aberrant expression of these genes underpins the association. For instance, a new variant associated with decreased risk of IBD increases the expression of VSIR in colon Fig4.

VSIR knockout mice have increased expression of IL23 and develop chronic inflammation in multiple tissues. Fine-mapping analyses identified likely causal missense variants at three new loci. DOK2 (ORCD=1.3, 27p=2x10-12) encodes a protein expressed in macrophages and T cells. Loss of Dok2 in mice causes severe DSS-induced colitis with reduced IL17A and IL22 expression. The same variant has been recently associated with CD in a sequencing study. SHARPIN (ORCD=1.2, p=1x10-16) is part of the linear ubiquitin chain assembly complex that modulates activation of the NF-κB pathway. Loss-of-function (LOF) mutations in other proteins in the complex are associated with immunodeficiency and systemic autoinflammation. CARMIL2 (ORUC=1.2, p=1x10-10) is required for NF-κB signalling in both B and T cells. LOF mutations are associated with primary immunodeficiencies and paediatric forms of IBD.
ConclusionThe greatly expanded sample size in our latest GWAS meta-analysis has enabled the identification of low frequency variants with larger effects on IBD susceptibility than the more common variants typically identified by previous GWAS. The biological overlap between Mendelian and complex forms of IBD is demonstrated by the discovery of common non-coding variants associated with complex forms of IBD that dysregulate the function of a Mendelian IBD gene.
Expectations of histopathologistsYear: 2017
Source: Talking Heads
Authors: Paula Borralho Nunes, Magali Svrcek
Created: Friday, 22 February 2019, 3:31 PM by ECCO Administrator
Last Modified: Wednesday, 2 June 2021, 11:44 AM by ECCO Administrator
Exploring disease control by combining clinical, biological, and health-related quality of life remission with endoscopic improvements among Ulcerative Colitis patients treated with filgotinib: A post-hoc analysis from the SELECTION trialYear: 2022
Source: ECCO'22 Virtual
Authors: Stefan Wolfgang Schreiber
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundFor patients with ulcerative colitis (UC), both subjectively and objectively reported measures are equally important treatment goals. We explored clinical, biological, HRQoL remission and endoscopic improvements as a combined endpoint (CE) from SELECTION (NCT02914522), a phase 2b/3 double-blind trial which assessed the efficacy and safety of filgotinib, a once-daily, oral, Janus 1 kinase preferential inhibitor, for the treatment of UC.
MethodsIn SELECTION, patients with moderately to severely active UC were randomized 2:2:1 to 200mg filgotinib (FIL200), 100mg filgotinib, or placebo (PBO) for an 11-week induction phase followed by a 47-week maintenance period in patients who achieved clinical remission or response.1 We defined a CE as achieving all of the following: 1) clinical remission defined as partial Mayo Score (excluding endoscopy domain) ≤2 and no sub-score >1), 2) biological remission defined as faecal calprotectin <150 µg/g, 3) Inflammatory Bowel Disease Questionnaire (IBDQ) remission defined as IBDQ >170 and 4) endoscopic improvement defined as Mayo endoscopic sub-score ≤1. We evaluated the CE among patients treated with FIL200 vs placebo in induction (week 10) and maintenance (week 58) phases. Among those achieving the CE, we analysed MCID improvement during induction and decline during maintenance on generic QoL instruments (36-item short-form questionnaire and EuroQol 5-dimension [EQ5D]).2,3,4
ResultsThe overall population included 381 biologic-naïve and 401 biologic-experienced patients undergoing induction, of which 297 subsequently entered maintenance. A higher proportion of patients receiving FIL200 achieved CE than PBO by week 10 in the biologic-naïve induction cohort (17.6% vs 4.41%, p<0.001) and by week 58 in the maintenance cohort (22.1% vs 7.14%, p=0.002) (Table 1). Biologic-naïve CE achievers had higher MCID improvement across all generic QoL scales and domains (Table 2).Patients achieving CE in maintenance experienced a lower proportion of MCID decline in EQ5D utility, and visual analogue scale.

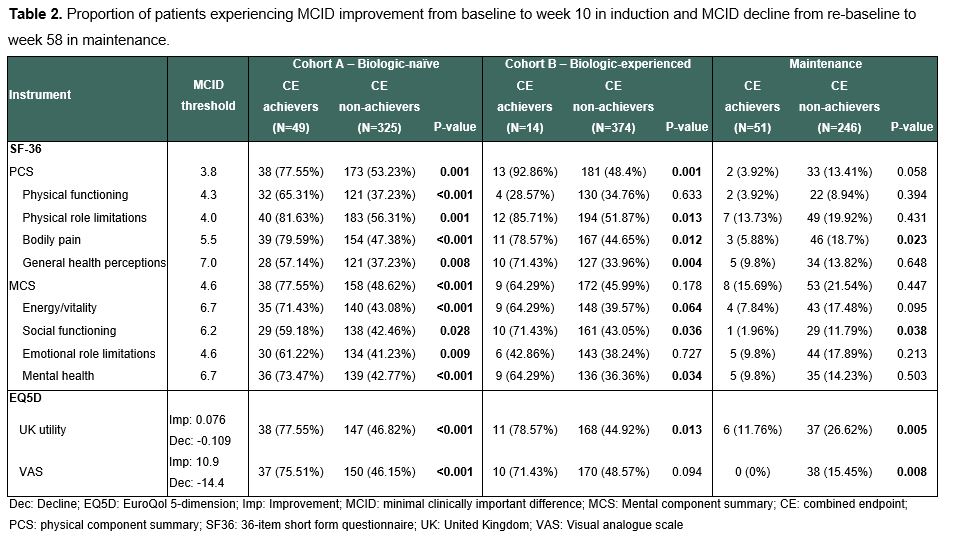
ConclusionTreatment with filgotinib resulted in a higher proportion of patients with combined clinical, biological, HRQoL remission and endoscopic improvements. Among patients achieving this combined composite endpoint, clinically meaningful improvements were observed in their overall quality of life. Holistic assessment of several subjective and objective measures may help achieve better outcomes in UC.
1. Feagan BF, et al. Lancet 2021;397:2372–84.
2. User’s manual for the sf-36v2 health survey. Quality Metric, Inc; 2009.
3. Stark RG, et al. Inflamm Bowel Dis. 2010 Jan;16(1):42–51.
4. Irvine EJ. J Pediatr Gastroenterol Nutr. 1999 Apr;28(4):S23–27.
Exposome Year: 2018
Source: 16th IBD Intensive Advanced Course
Authors: Halfvarson Jonas
Created: Tuesday, 8 May 2018, 11:36 AM
Files: 1
.png)
.png)





