Exabis Library
Welcome to the e-CCO Library!
Immunological effects of nutritional interventions in IBD
2019
4th D-ECCO Workshop
Wednesday, 5 June 2019, 9:01 PM
Immunological effects of nutritional interventions in IBD
2019
4th D-ECCO Workshop
Tuesday, 28 May 2019, 3:32 PM
1
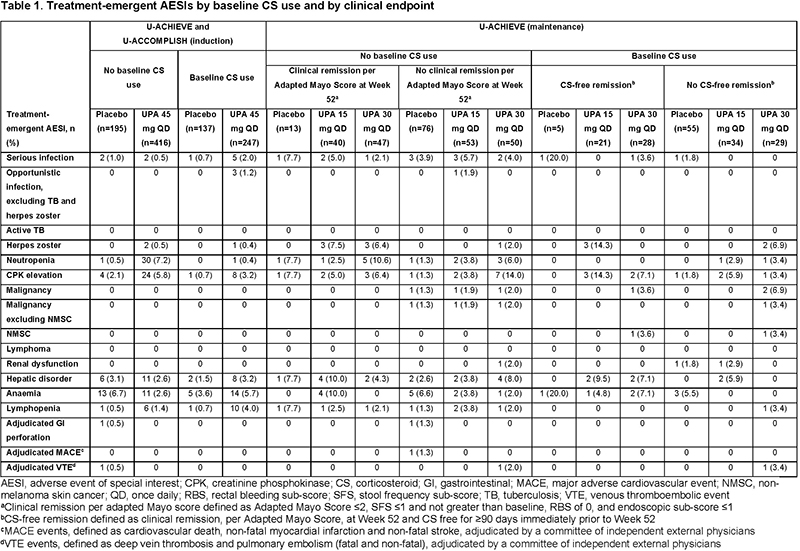
Impact of corticosteroid usage on efficacy and safety outcomes in patients receiving upadacitinib for Ulcerative Colitis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Impact of COVID-19 on clinical research in IBD
2022
8th ClinCom Workshop
Tuesday, 24 May 2022, 8:13 PM
In-depth characterisation of the serum antibody epitope repertoire in Inflammatory Bowel Disease by high-throughput phage-displayed immunoprecipitation sequencing
2022
8th Y-ECCO Basic Science Workshop
Tuesday, 24 May 2022, 8:13 PM
In-depth characterisation of the serum antibody epitope repertoire in Inflammatory Bowel Disease by high-throughput phage-displayed immunoprecipitation sequencing
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Increasing incidence of Inflammatory Bowel Disease in a high prevalence country: A nationwide study in Finland
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Increasing incidence of pouchitis among patients undergoing ileal pouch-anal anastomosis between 1996 and 2018: A population-based Danish cohort study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Infections with biologics and small molecules: Impact on drug positioning
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Inflammatory Bowel Disease (IBD) and Solid Organ Transplantation. Natural history of pre-existing and de novo IBD patients. (EITOS study of GETECCU)
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Inflammatory pathways
2017
15th IBD Intensive Advanced Course
Wednesday, 15 March 2017, 1:13 PM by Vesna Babaja
1
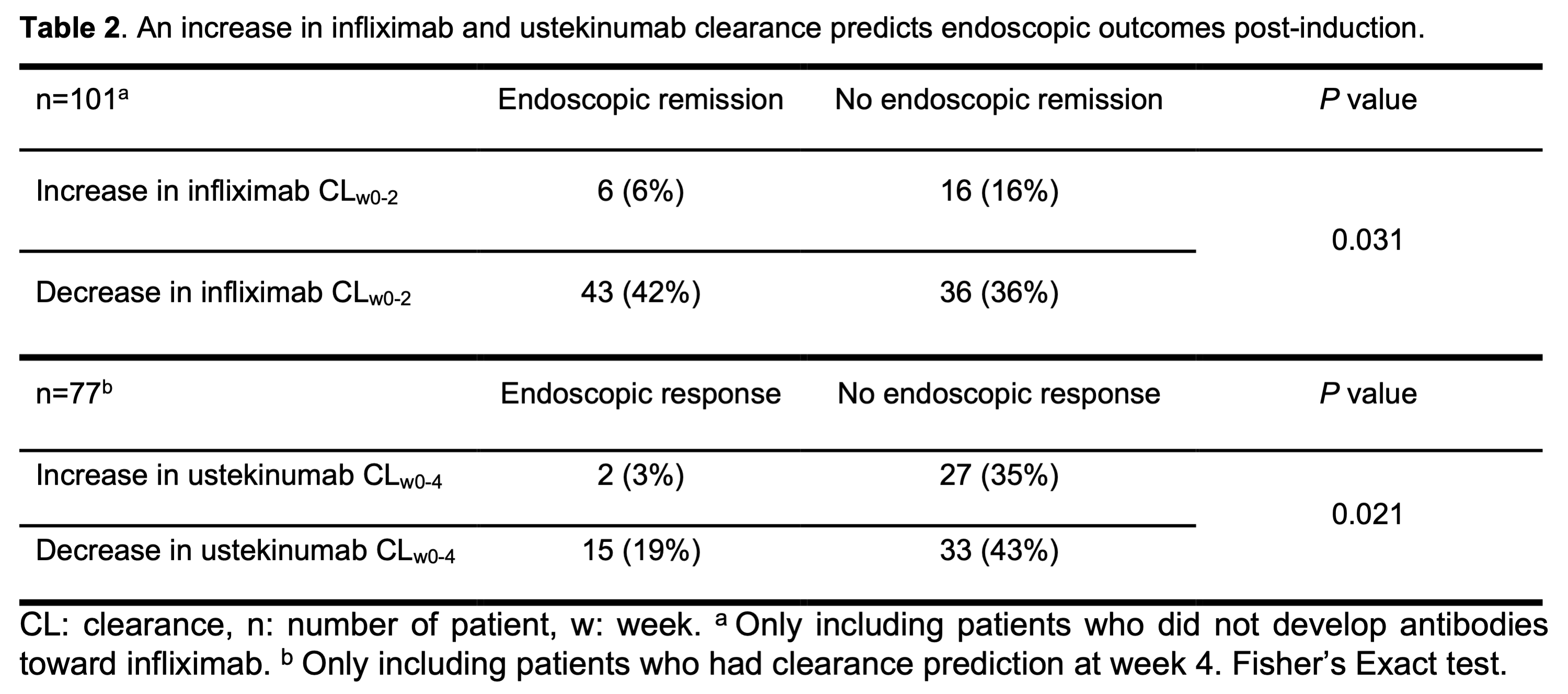
Infliximab and ustekinumab clearance during induction predicts post-induction endoscopic outcomes in patients with Crohn’s Disease
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Initial work up after diagnosis of IBD (Tandem talk)
2022
6th Basic ECCO: EduCational COurse for Industry
Tuesday, 24 May 2022, 8:13 PM
Integrated tissue transcriptomic and serum proteomic interrogation reveals biomarkers for endoscopic improvement and histologic remission after JAK3/TEC inhibition with ritlecitinib (PF-06651600) in Ulcerative Colitis (UC) (Phase 2b Vibrato study)
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM



 Conclusion
Conclusion