OP38: Tofacitinib for the treatment of Ulcerative Colitis: An integrated summary of safety data from the global OCTAVE and RIVETING clinical trialsYear: 2022
Source: ECCO'22
Authors: Sandborn, W.J.(1);D'Haens, G.R.(2);Sands, B.E.(3);Panaccione, R.(4);Ng, S.C.(5);Lawendy, N.(6);Kulisek, N.(6);Guo, X.(6);Mundayat, R.(7);Su, C.(7);Panés, J.(8);
Created: Friday, 11 February 2022, 3:52 PM
OP39: Safety of ustekinumab in IBD: Final pooled long-term safety analysis through 5 years in CD and 4 years in UCYear: 2023
Source: ECCO’23 Copenhagen
Authors: Ghosh, S.(1)*;Feagan, B.(2);Ott, E.(3);Gasink, C.(3);Marano, C.(4);Miao, Y.(4);Sandborn, W.(5);Danese, S.(6);Abreu, M.(7);Sands, B.(8);
Created: Friday, 14 July 2023, 10:43 AM
OP39: Shorter disease duration is associated with better outcomes in patients with moderately to severely active Crohn’s Disease treated with risankizumab: Results from the phase 3 ADVANCE, MOTIVATE, and FORTIFY studiesYear: 2022
Source: ECCO'22
Authors: Peyrin-Biroulet, L.(1);Colombel, J.F.(2);Louis, E.(3);Ferrante, M.(4);Motoya, S.(5);Panaccione, R.(6);Torres, J.(7);Ungaro, R.C.(2);Kligys, K.(8);Kalabic, J.(9);Zambrano, J.(8);Zhang, Y.(8);D’Haens, G.R.(10);
Created: Friday, 11 February 2022, 3:52 PM
OP39: The effect of phenotype and genotype on the plasma proteome in patients with Inflammatory Bowel DiseaseYear: 2021
Source: ECCO'21 Virtual
Authors: Bourgonje, A.R.(1);Hu, S.(1);Spekhorst, L.M.(1);Zhernakova, D.V.(2);Vich Vila, A.(1);Li, Y.(1);Voskuil, M.D.(1);van Berkel, L.A.(3);Bley Folly, B.(3);Charrout, M.(4);Mahfouz, A.(4);Reinders, M.J.T.(4);van Heck, J.I.P.(5);Joosten, L.A.B.(5);Visschedijk, M.C.(1);van Dullemen, H.M.(1);Faber, K.N.(1);Samsom, J.N.(3);Festen, E.A.M.(1);Dijkstra, G.(1);Weersma, R.K.(1)
Created: Wednesday, 2 June 2021, 4:12 PM
OP39: The effect of phenotype and genotype on the plasma proteome in patients with Inflammatory Bowel DiseaseYear: 2021
Source: ECCO'21 Virtual
Authors: Arno Bourgonje
Created: Friday, 1 October 2021, 12:41 PM
BackgroundProtein profiling in patients with inflammatory bowel diseases (IBD) for diagnostic and therapeutic purposes is underexplored. Assessment of interactions between genetics and the plasma proteome could lead to identification of novel disease-associated molecular pathways. In this study, we performed the largest gene-protein association analysis thus far in patients with IBD, taking into account relevant phenotypic covariates and integrating information from multiple biological data layers.
MethodsNinety-two (92) inflammation-related proteins were quantified in plasma of 1,028 patients with IBD (567 Crohn’s disease [CD]; 461 ulcerative colitis [UC]) and 148 healthy individuals to assess proteome-phenotype associations. Both whole-exome sequencing (WES) and global screening array (GSA) data of 919 patients with IBD were included to study associations between over 8 million genetic variants and protein levels (protein quantitative trait loci [pQTL]). Cis-pQTLs were defined within ± 1 Mb of the region of each protein-coding gene center, whereas trans-pQTLs were outside of that region. After adjusting for phenotypic covariates, a step-wise conditional analysis was used to identify all independent pQTLs in CD and UC separately, followed by a meta-analysis. Intestinal mucosal RNA sequencing and fecal metagenomic data were used for complementary analyses.
ResultsThirty-four (34) proteins were differentially abundant between IBD and healthy individuals, of which 24 proteins independent of active inflammation. (Figure 1) Seventy-two (72) proteins were significantly associated to 14 phenotypic factors, including age, sex, medication use, and surgical history. (Figure 2) Fibroblast growth factor-19 (FGF-19) levels were decreased in CD patients with ileal disease or a history of ileocecal resection. Thirteen (13) novel cis-pQTL variants were identified and 10 replicated from previous studies, together affecting 21 different plasma proteins. One trans-pQTL variant of the FUT2 gene (rs602662) and two independent cis-pQTL variants of the CCL25 gene significantly affected plasma C-C motif chemokine ligand 25 (CCL25) levels. (Figure 3) Intestinal gene expression data revealed an overlapping cis-expression (e)QTL-variant (rs3745387) of the CCL25 gene. The FUT2 rs602662 trans-pQTL variant associated significantly with reduced abundances of multiple fecal butyrate-producing bacteria, including the genus Blautia and the species Faecalibacterium prausnitzii.



ConclusionThis study shows that both genotype and multiple disease phenotypes strongly associate with the plasma proteome in patients with IBD and identifies disease-associated pathways that may help to improve disease management in the future.
OP40 A core transferable microbiota in responders to faecal microbiota transplant for ulcerative colitis shape mucosal T-cell immunityYear: 2020
Authors: L. Gogokhia1, S. Lima1, M. Viladomiu1, Y. Gerardin2, C. Crawford1, V. Jacob1, E. Scherl1, M. Rosenthal3, S.E. Brown3, J. Hambor3, R. LONGMAN1
Created: Thursday, 30 January 2020, 10:12 AM
OP40: Analysis of clinical features associated with favourable outcomes from ustekinumab treat-to-target strategy in Crohn’s Disease patients in the STARDUST trialYear: 2021
Source: ECCO'21 Virtual
Authors: Danese, S.(1);Vermeire, S.(2);Dignass, A.(3);Panés, J.(4);D'Haens, G.(5);Magro, F.(6,7);Le Bars, M.(8);Nazar, M.(9);Lahaye, M.(10);Ni, L.(11);Bravatà, I.(12);Gaya, D.R.(13);Peyrin-Biroulet, L.(14)
Created: Wednesday, 2 June 2021, 4:12 PM
OP40: Analysis of clinical features associated with favourable outcomes from ustekinumab treat-to-target strategy in Crohn’s Disease patients in the STARDUST trialYear: 2021
Source: ECCO'21 Virtual
Authors: Silvio Danese
Created: Friday, 1 October 2021, 12:41 PM
BackgroundThe 48-week (W) interventional STARDUST trial assessed whether a treat-to-target (T2T) strategy using ustekinumab (UST) may optimize Crohn’s disease (CD) outcomes; primary efficacy and safety data have been reported before.1 Here we assessed which patient (pt) subgroups may benefit from T2T vs standard of care (SoC) in achieving endoscopic response after 1 year of UST treatment.
MethodsAdult pts with moderate–severely active CD (CD activity index [CDAI] 220–450) and Simple Endoscopic Score in CD [SES-CD] ≥3) who failed conventional therapy and/or 1 biologic were included. Pts received iv, weight-based UST ~6 mg/kg at W0 (baseline [BL]); then SC UST 90 mg at W8. At W16, CDAI 70 responders were randomized (1:1) to T2T or SoC arms. Pts in the T2T arm were assigned to SC UST q12w or q8w based on 25% improvement in SES-CD score vs BL. From W16–48, UST dose was further intensified up to q4w if the following were not met: CDAI <220 and ≥70-point improvement from BL, and C-reactive protein ≤10 mg/L or faecal calprotectin (FCal) ≤250 µg/g. Pts who failed treatment target despite UST q4w were discontinued. In the SoC arm, UST dose was assigned based on EU SmPC (q12w or q8w). We report the treatment effect for the primary endpoint (endoscopic response [≥50% improvement in SES-CD score vs BL] at W48), evaluated for subgroups of pts, based on demographics at BL. For each subgroup, the odds ratio (OR) and 95% confidence interval (CI) of T2T vs SoC were provided based on the logistic regression model that included treatment arm and stratification factors (prior exposure to biologics [none or 1] and SES-CD score [≤16, >16] at BL) as independent variables.
ResultsOf 500 pts enrolled, 441 were randomized to T2T (n=220) or SoC (n=221); 79.1% and 87.3% completed W48. At W48, pts randomized to T2T were more likely to achieve endoscopic response compared to SoC (p<0.05), if they had at BL: (i) longer disease duration (>median [79.1 months]; OR 2.2; 95%CI 1.17–3.94); (ii) clinically moderate disease (CDAI ≤300; OR 1.7; 95%CI 1.03–2.76); (iii) normal FCal (≤250; OR 3.0; 95%CI 1.22–7.56), (iv) endoscopically active CD (SES-CD ≥4 for ileal or ≥6 for colonic and/or ileocolonic disease; OR 1.8; 95%CI 1.10–2.91); and (v) history or presence of strictures/fistula or occurrence of an intra-abdominal abscess (OR 2.3; 95%CI 1.06–5.01 and OR 3.5; 95%CI 1.07-11.19, respectively; Figure 1).
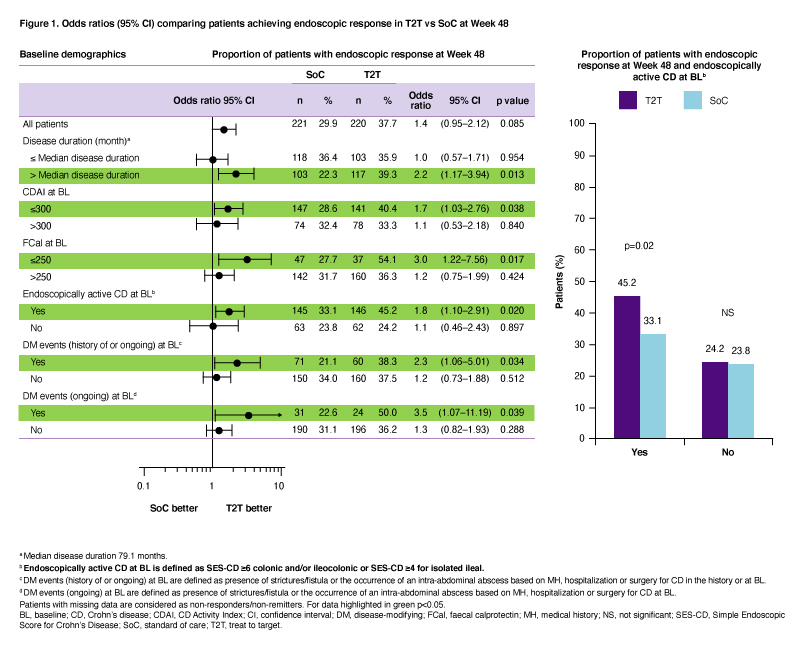
ConclusionT2T was more effective than SoC (p<0.05) in achieving endoscopic response after 1 year of UST treatment in certain subgroups including pts with higher endoscopic scores at BL and those with history/presence of bowel damage.
1. Danese S, et al. United European Gastroenterol J. 2020;8:1264–1265 (Abstract LB11).
OP40: Efficacy of risankizumab induction and maintenance therapy by baseline Crohn’s Disease location: Post hoc analysis of the phase 3 ADVANCE, MOTIVATE, and FORTIFY studiesYear: 2022
Source: ECCO'22
Authors: Bossuyt, P.(1);Bresso, F.(2);Dubinsky, M.(3);Ha, C.(4);Siegel, C.(5);Zambrano, J.(6);Kligys, K.(6);Kalabic, J.(6);Zhang, Y.(6);Panaccione, R.(7);
Created: Friday, 11 February 2022, 3:52 PM
OP40: PRA023 Demonstrated Efficacy and Favorable Safety as Induction Therapy for Moderately to Severely Active UC: Phase 2 ARTEMIS-UC Study ResultsYear: 2023
Source: ECCO’23 Copenhagen
Authors: B. Sands*(1), L. Peyrin-Biroulet(2), S. Danese(3), D.T. Rubin(4), S. Vermeire(5), O. Laurent(6), A. Luo(6), D. Nguyen(6), JD. Lu(6),A. Wiechowska-Kozlowska(7), J. Leszczyszyn(8), R. Kempinski(9), J. Kierkus(10), C. Ma(11), T. Ritter*(12), B.G. Feagan(13), S. Targan*(14)
Created: Friday, 14 July 2023, 10:43 AM



