Exabis Library
Welcome to the e-CCO Library!
A case for and against oral iron in IBD: Tandem talk
2018
3rd D-ECCO Workshop
Tuesday, 8 May 2018, 11:36 AM
Tuesday, 8 May 2018, 3:44 PM by Lindley Fritze
1
A comparative efficacy and safety analysis of subcutaneous infliximab and vedolizumab in patients with Crohn’s Disease and Ulcerative Colitis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
A multi-centre study to explore the prevalence of Inflammatory Bowel Disease indicators in patients with moderate- to- severe psoriasis: Baseline results from the EPIC study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
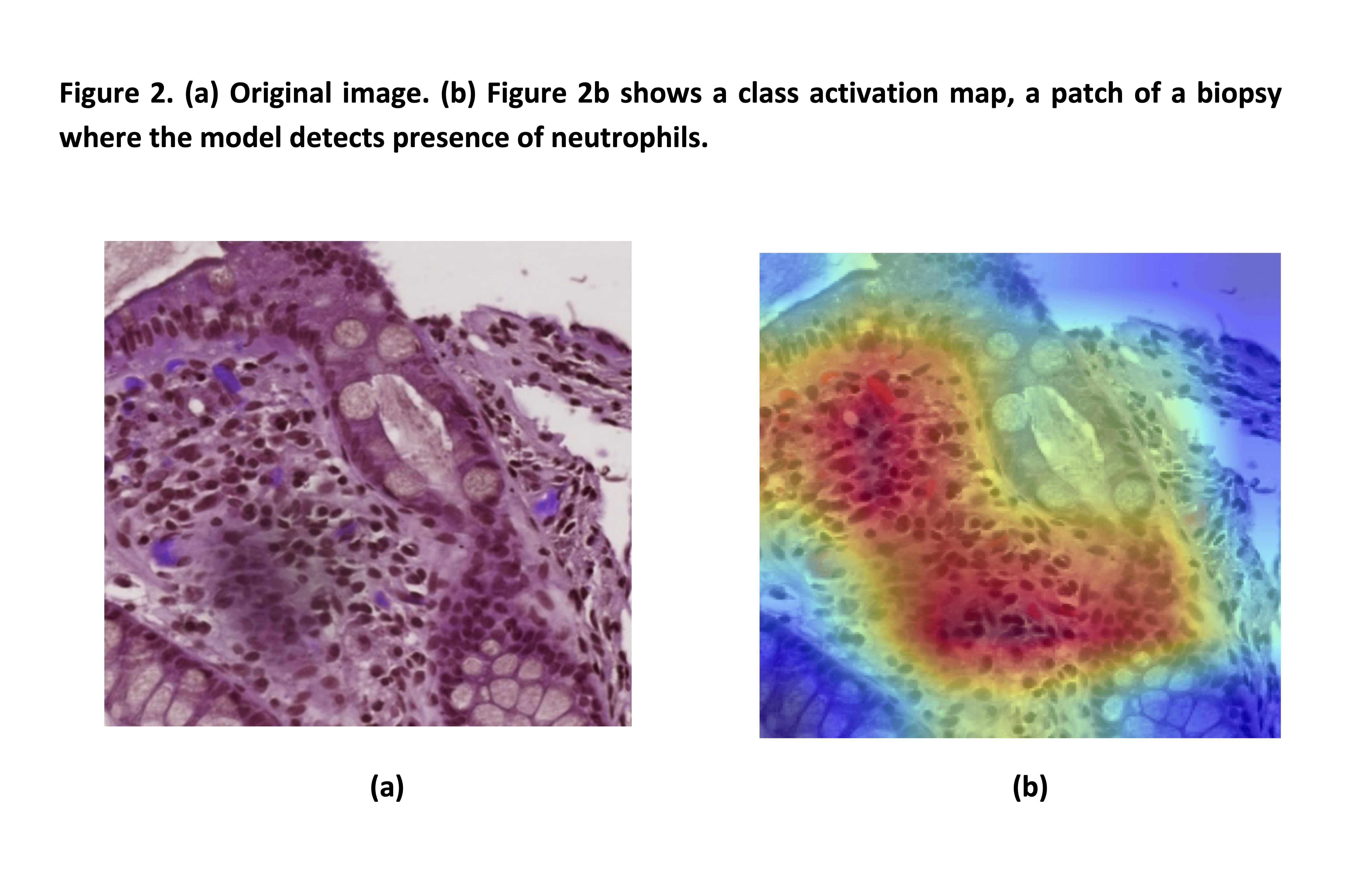
A new simplified histology artificial intelligence system for accurate assessment of remission in Ulcerative Colitis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
A novel predictive scoring system of venous thromboembolism after surgery for Inflammatory Bowel Disease: A NSQIP-IBD registry analysis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
A Phase 1 Study Evaluating the Bioequivalence of the Proposed Commercial and Clinical Formulations of Etrasimod 2mg, and the Effect of Food on the Pharmacokinetics of the Proposed Commercial Formulation in Healthy Volunteers
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
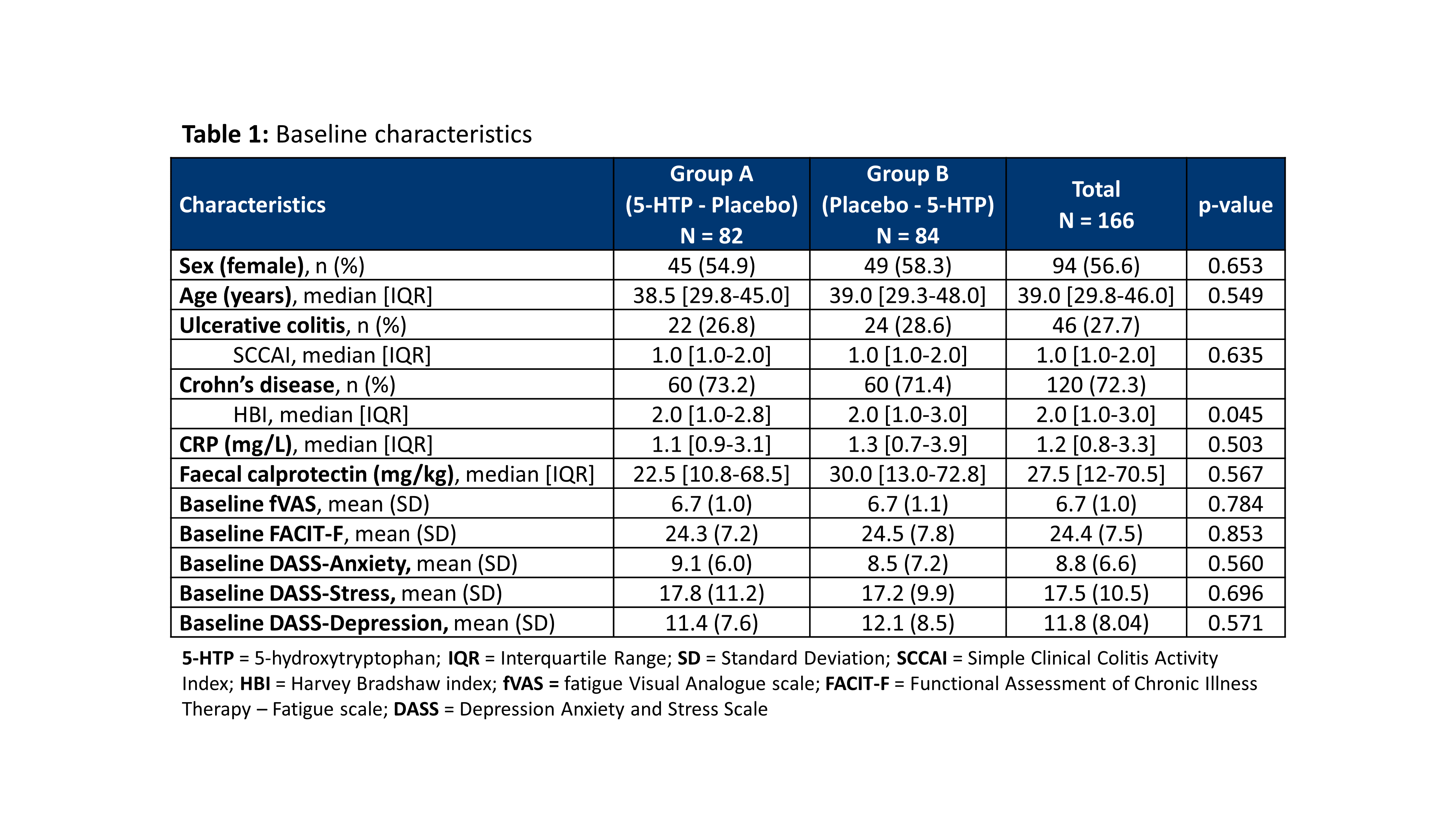
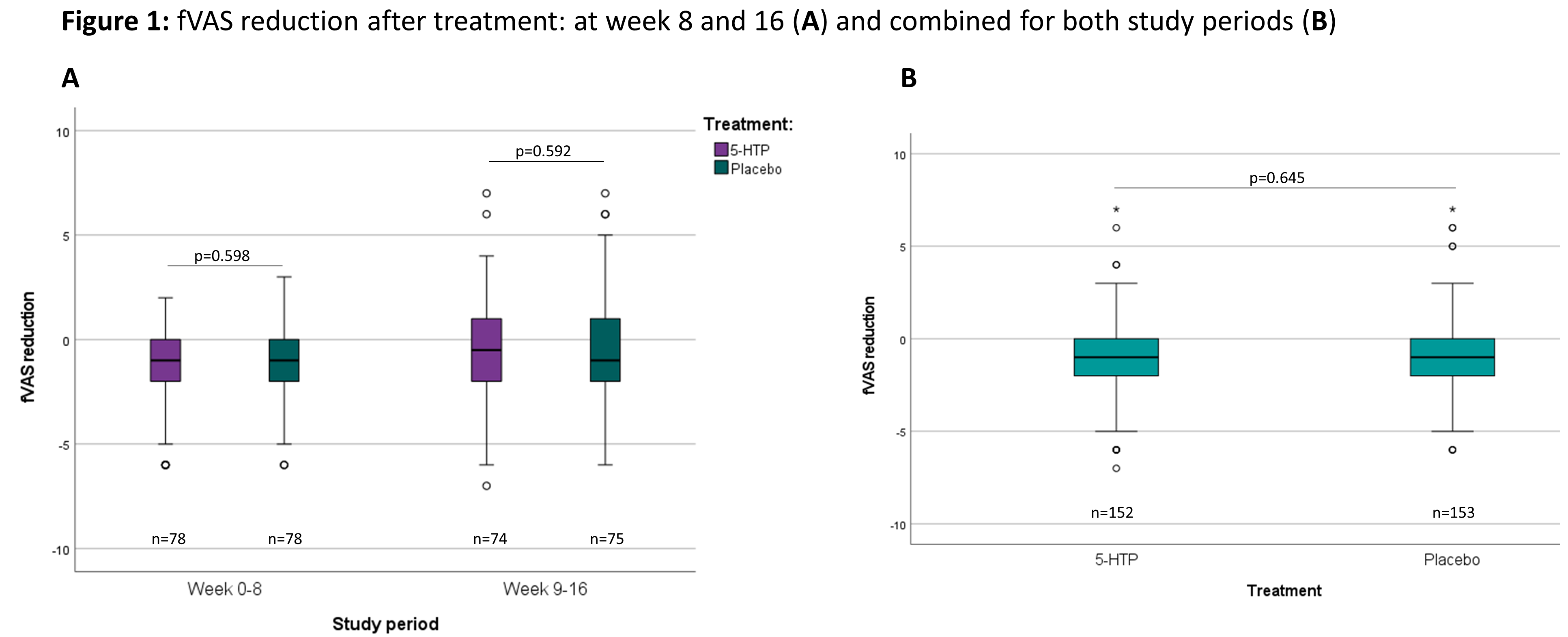
A randomized placebo controlled clinical trial with 5-hydroxytryptophan in patients with quiescent Inflammatory Bowel Disease and fatigue (Trp-IBD)
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
A rectovaginal fistula complicating CD
2019
8th S-ECCO IBD Masterclass
Wednesday, 5 June 2019, 9:01 PM
A rectovaginal fistula complicating CD
2019
8th S-ECCO IBD Masterclass
Tuesday, 28 May 2019, 3:32 PM
1
A refractory proctitis complicating CD
2019
8th S-ECCO IBD Masterclass
Wednesday, 5 June 2019, 9:01 PM
A refractory proctitis complicating CD
2019
8th S-ECCO IBD Masterclass
Tuesday, 28 May 2019, 3:32 PM
1
A systematic review of the impact of Inflammatory Bowel Disease (IBD) on family members
2022
16th N-ECCO Network Meeting
Tuesday, 24 May 2022, 8:13 PM
Abstract 1: Developing a colorectal cancer risk prediction tool for patients with Ulcerative Colitis and low grade dysplasia
2018
7th S-ECCO IBD Masterclass
Tuesday, 8 May 2018, 11:36 AM
1
Abstract 1: Epigenetic alterations at diagnosis predict susceptibility, prognosis and treatment escalation in Inflammatory Bowel Disease - IBD Character
2017
3rd Y-ECCO Basic Science Workshop
1
Abstract 1: Iterative ileocolonic resection for Crohn's Disease: A prospective multicentric cohort study of the GETAID Chirurgie
2019
8th S-ECCO IBD Masterclass
Wednesday, 5 June 2019, 9:01 PM
Abstract 1: Iterative ileocolonic resection for Crohn's Disease: A prospective multicentric cohort study of the GETAID Chirurgie
2019
8th S-ECCO IBD Masterclass
Tuesday, 28 May 2019, 3:32 PM
1






 Conclusion
Conclusion