Combining therapies: Pros and consYear: 2021
Source: ECCO'21 Virtual
Authors: David Rubin
Created: Friday, 1 October 2021, 12:41 PM
Summary contentObjectives:
1. To conceptualize the chronic management of complex IBD
2. To develop a multi-phased approach to combination therapy in IBD
3. To consider future strategies of management
Summary:
The limitations of current treatments for IBD demand new approaches to management, including novel combinations of therapies. Combination approaches should consider multiple mechanisms, sequencing and de-escalation options. Clinical trials of novel approaches require creative and precision medicine-based strategies to demonstrate efficacy and safety as well as potential cost effectiveness.
Communicating risks – IBD research nurse and physician perspective (Tandem Talk)Year: 2021
Source: 4th School for Clinical Trialists
Authors: Peter Bossuyt, Katrien Asnong
Created: Friday, 1 October 2021, 12:41 PM
Summary contentEducational objectives:
1. To understand how risks in clinical trials are defined.
2. To review the way how the risk of clinical trials is communicated by the HCP to the patient and the shortcommings.
3. To understand the factors that influence the perception by the patient of the communicated risk.
4. To have insight in tips and tricks for an optimal communication of risk in a clinical trial setting.
Comparative effectiveness of vedolizumab and ustekinumab in Crohn’s Disease patients who failed anti-TNF treatment: Interrogating 1019 patients from the UK IBD BioResourceYear: 2022
Source: ECCO'22 Virtual
Authors: Rofaida Desoki
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundUstekinumab (UST) and vedolizumab (VDZ) are widely used to treat patients with Crohn’s disease (CD). However, limited data exist regarding comparative effectiveness of these agents for patients with CD who have failed anti-TNF treatment. We aimed to compare the efficacy of UST and VDZ utilizing the largest cohort of CD patients who failed anti-TNF in real world clinical practice.
MethodsWe conducted a retrospective cohort analysis using data retrieved from the UK IBD BioResource, capturing 34,148 subjects. We identified patients with CD, who failed anti-TNF and were subsequently treated with UST or VDZ as second or third-line therapy. Inverse probability of treatment weighting (IPTW) was used to balance groups using a propensity score-weighting approach accounting for baseline patient or disease related characteristics. Persistence on therapy with clinician assessment of treatment success, without the need for treatment change or surgery was used to estimate the response to treatment. We compared treatment survival curves before and after IPTW and used a log rank test for differences between groups
Results654 CD patients received VDZ, either as second line (51%) or third line (49%) therapy. 365 patients received UST, 52% as a second line and 48% as a third line therapy. All patients received either infliximab or adalimumab as first and/or second biologic therapy. Baseline characteristics are detailed in table 1. Following IPTW, variables were well balanced. Patients receiving VDZ showed similar rates of treatment success compared to UST as second- and third-line biologic agent after anti TNF failure (before IPTW adjustment, log rank p 0.241; after IPTW, log rank p 0.154). Outcomes for UST were similar between 2nd and 3rd line usage (p 0.81), but outcomes for VDZ were significantly worse when used 3rd line compared to 2nd line (p <0.0001).Subgroup analysis of unadjusted survival data showed significantly better outcomes for patients with ileal disease distributiontreated with UST compared to VDZ (p=0.043) but no significant differences in outcomes for subgroups with colonic or ileocolonic disease.We estimate persistence on UST and VDZ to be 67%, 54%, 49% and 49% at 1, 2, 3 and 5 years respectively.
ConclusionUsing data from a multi-institutional cohort of patients with CD with larger number of participants and longer follow-up than previous cohorts, we demonstrate no difference between UST and VDZ used as second and/or third line biologic therapy, after anti-TNF failure. Subgroup analysis reveals some patient characteristics predictive of differential treatment response.



Comparative efficacy of biologics for endoscopic healing of the ileum and colon in Crohn’s DiseaseYear: 2022
Source: ECCO'22 Virtual
Authors: Neeraj Narula
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundWe compared the efficacy of adalimumab, infliximab, ustekinumab, and vedolizumab for achieving endoscopic healing (EH) in the ileum and colon after one-year of therapy in Crohn’s disease (CD).
MethodsA pooled analysis of patient-level data from 344 patients with CD from four clinical trial programs was performed. Patients who received continuous adalimumab, infliximab, ustekinumab, or vedolizumab throughout the trial and had at least one ileocolonic segment with a Simple Endoscopic Score for CD (SES-CD) ≥ 3 at enrolment were included. Proportions of patients achieving one-year endoscopic healing (EH), defined as SES-CD of 0, using each of four biologics were compared. Multivariate logistic regression was used to model the relationship between individual biologics and one-year outcomes, adjusted for potential confounders of EH, including disease duration, concomitant corticosteroid use, and prior anti-TNF failure.
ResultsCompared to vedolizumab [10/77 (13%)], both infliximab [29/79 (36.7%), aOR: 3.27 (95% CI: 1.34-8.01), p<0.001] and adalimumab [12/40 (30%), aOR: 3.01 (95% CI: 1.10-8.21), p=0.032] were superior for achieving one-year EH of the ileum among patients with ileal involvement at baseline. No difference was observed between ustekinumab [5/22 (22.7%)] and vedolizumab [aOR: 2.75 (95% CI: 0.76-9.91), p=0.123]. In biologic-naïve patients, ustekinumab, adalimumab, and infliximab were superior to vedolizumab for achieving one-year EH of the ileum. For colonic disease, in comparison to ustekinumab [9/31 (29.0%), adalimumab [30/48 (62.5%), aOR: 4.04 (95% CI: 1.88-8.71), p<0.001] and infliximab (55/105 (52.4%), aOR: 2.02 (95% CI: 1.03-3.99), p=0.041] were superior for one-year EH in the colon among patients with colonic involvements at baseline. No difference was seen between vedolizumab [26/87 (29.9%)] and ustekinumab [aOR: 1.01 (95% CI: 0.39-2.59), p=0.987]. Similar differences were noted among biologic-naïve patients.



 Conclusion
ConclusionIn this post-hoc analysis of pivotal clinical trials, TNFα antagonists were generally superior to vedolizumab and ustekinumab for achieving EH of the ileum and colon in patients with CD. However, among biologic-naïve patients, ustekinumab, adalimumab, and infliximab were superior to vedolizumab for attaining one-year EH of the ileum.
Comparative real-world effectiveness and persistence of vedolizumab versus anti-TNF therapy in biologic-naïve patients with Crohn´s Disease with Propensity Score adjustment: Maintenance phase results at week-52 from the prospective VEDOIBD studyYear: 2022
Source: ECCO'22 Virtual
Authors: Bernd Bokemeyer
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundTo gain insight into vedolizumab (VDZ) use as a first-line biologic in Crohn´s Disease (CD), this real-world study aimed to assess, within the maintenance phase, the 1-year comparative effectiveness and persistence of VDZ vs anti-TNF therapy in biologic-naïve CD-patients.
MethodsBetween 2017-2020, 1200 consecutively enrolled biologic-naïve and biologic-experienced patients with ulcerative colitis (UC) and CD were prospectively included in the VEDOIBD-Registry from 45 IBD-experienced centres across Germany. 294 biologic-naïve CD-patients starting a new therapy with VDZ or anti-TNF (adalimumab: ADA or infliximab: IFX) were included in this real-world evidence (RWE) study. The Kaplan-Meier was used to summarize the treatment persistence from the start of therapy through week-52. The primary outcome was week-52 clinical remission (HBI ≤ 4). Patients were analyzed on a modified intent-to-treat basis (mITT; switchers considered as outcome failure) and on a per-protocol (PP) basis (excluding switchers). To reduce selection bias in the estimation of treatment effects, the inverse probability of treatment weighting propensity score (PS) was implemented. A weighted logistic regression was used to evaluate the effectiveness. The results were reported as odds ratio (OR) and 95% confidence interval (CI).
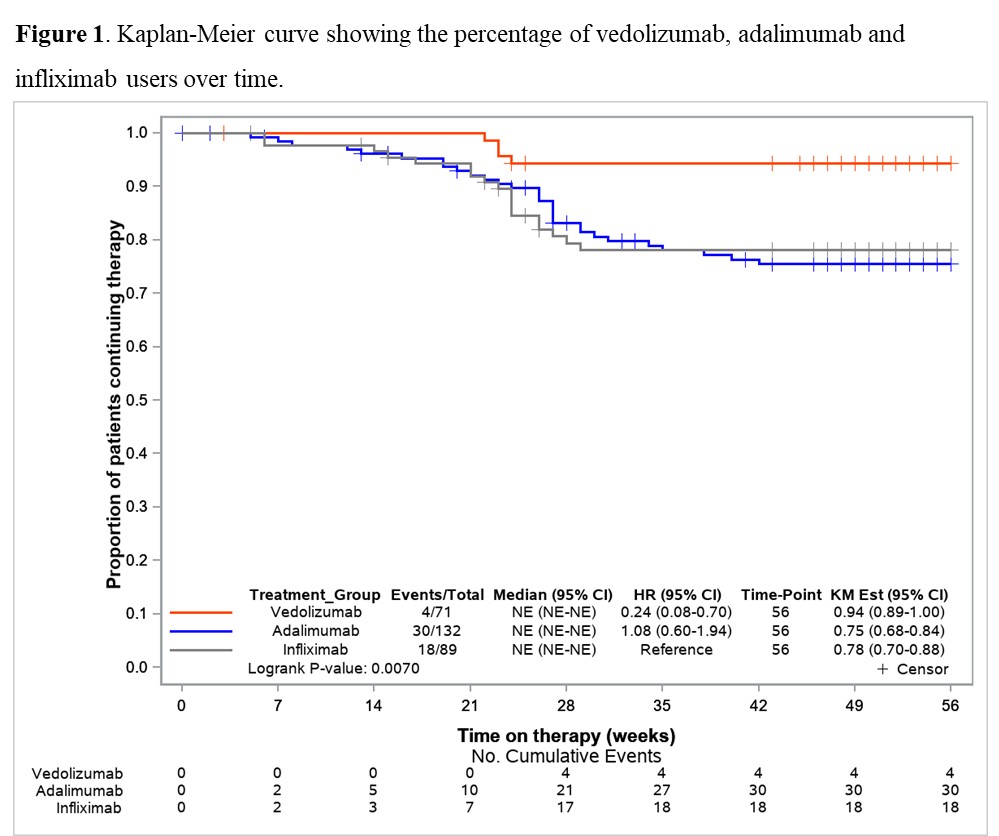
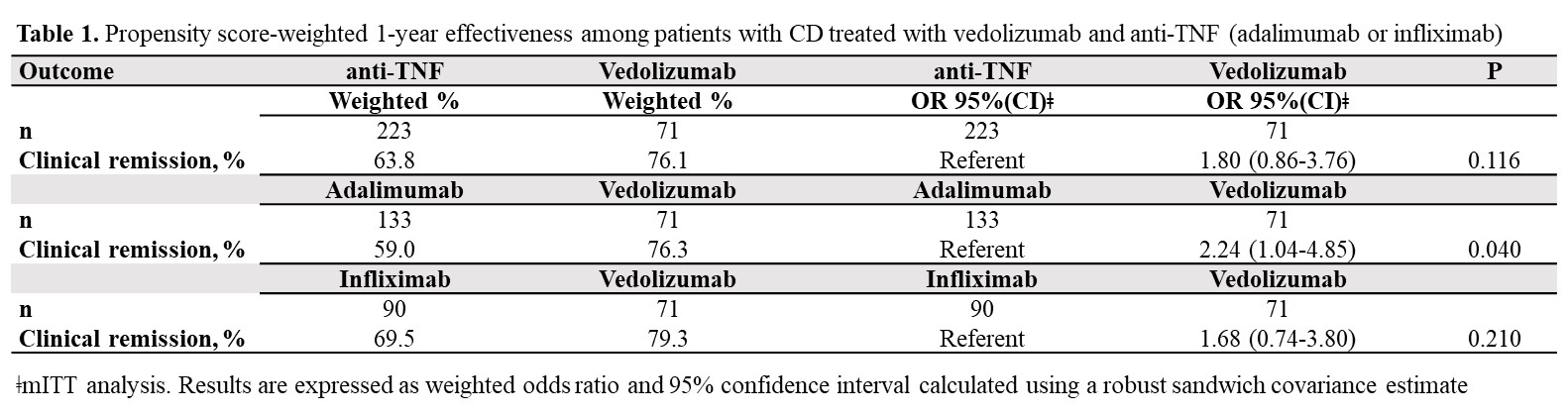
Results71 VDZ and 223 anti-TNF (ADA: 59.6%, IFX: 40.4%) biologic-naïve CD-patients were evaluated. 52-weeks after treatment initiation approximately 94% of VDZ patients were still in continuous treatment vs 75% of ADA and 78% of IFX (Figure 1). The mITT 1-year clinical remission rate was 76.1% for VDZ vs 63.8% for anti-TNF (OR: 1.80, 95% CI: 0.86-3.76). Similar results were observed for VDZ vs IFX (Table 1). In contrast, the clinical remission was significantly higher in the VDZ group than in the ADA group (OR: 2.24, 95% CI: 1.04-4.85). The PP analysis suggested comparative effectiveness, having excluded more anti-TNF switchers. 91.7% of week-14 responders VDZ patients were in clinical remission from week 14 through 52 vs 66.1% of anti-TNF patients (OR: 5.69, 95% CI: 1.66-19.5). Similar, significant, results were observed for VDZ vs ADA and for VDZ vs IFX (Table 2).
ConclusionIn this real-world setting comparing VDZ and anti-TNF in biologic-naïve patients via PS weighted analysis, VDZ showed especially in week-14 responders higher clinical remission rates in comparison to anti-TNF. The higher treatment persistence observed for VDZ, perhaps due to a more favourable safety profile vs anti-TNF, may be considered the main driver for the better effectiveness of VDZ at one year. These findings may aid physicians’ decision-making on the choice of VDZ as the first-line biologic for CD.

Comparative study of the effectiveness of vedolizumab versus ustekinumab after anti-TNF failure (VERSUS-CD)Year: 2022
Source: ECCO'22 Virtual
Authors: María José García
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundMain aim: To evaluate the retention rate of ustekinumab compared to vedolizumab in Crohn’s disease patients who failed anti-TNF therapy in clinical practice. Secondary aims: To compare the short-term and long-term effectiveness, and the safety of both treatments.
MethodsCrohn’s disease patients who had received either vedolizumab or ustekinumab after failure or intolerance to anti-TNF agents from ENEIDA registry were included. ENEIDA is a prospectively maintained database promoted by GETECCU. A total of 755 patients from 30 centres were included at time of data extraction. Clinical activity was classified based on Harvey-Bradshaw index both at short (during induction) and in the long-term. Kaplan-Meier curves, Cox regression models, inverse probability weighting and propensity matching score analyses were performed to compare both drugs and to identify predictive factors of treatment effectiveness and durability.
Results755 patients were included (195 in the vedolizumab cohort and 560 in the ustekinumab cohort). After a median of 20 months (IQR 7.4-30) of follow-up, the survival rate for ustekinumab therapy was higher than vedolizumab (Figure 1). The propensity matching score verified the differences between both therapies. The short-term proportion of patients on clinical remission, steroid-free remission and clinical response was also superior in the ustekinumab cohort (Figure 2). In the long-term, significant differences were observed 2 years after the beginning of the treatments, although no differences in clinical response and remission rates were detected in patients who achieved clinical response at week 16 between both cohorts. Vedolizumab was discontinued in 142 patients and ustekinumab in 185, mainly due to primary non-response (52% in the vedolizumab and 58% in the ustekinumab cohort) and loss of response (34% and 25%, respectively) despite the fact that 35% of the patients required intensification. The predictive factors associated to the discontinuation of the therapy are described in table 1. Adverse events were observed, overall, in 12% of the patients, without differences between both groups (Table 2). Following the discontinuation of the treatment with vedolizumab/ustekinumab, other biologic agents were prescribed in 56% of the patients, and 27% underwent surgery.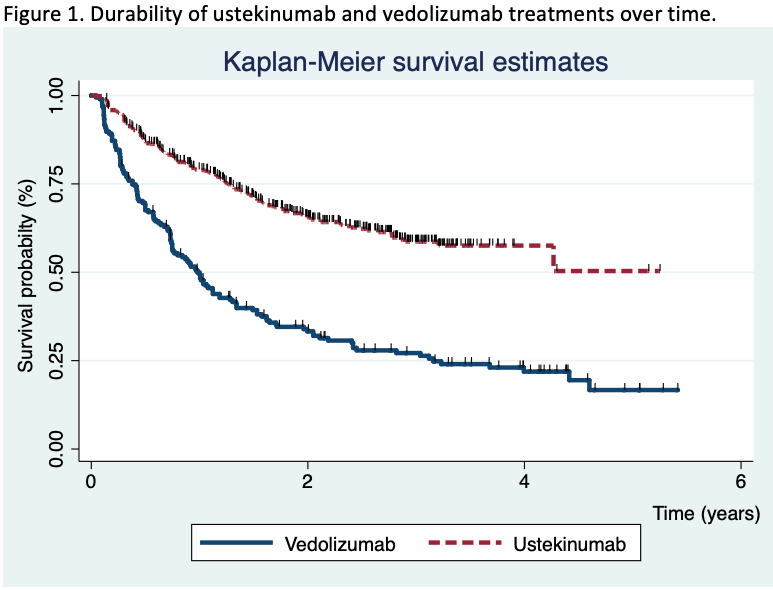



ConclusionIn clinical practice, a relatively high proportion of Crohn’s disease patients who received ustekinumab or vedolizumab for anti-TNF failure, maintained these drugs in the medium-long term, although ustekinumab retention rate was higher in comparison with vedolizumab.
Comparison of the risk of clinical recurrence after ileocolonic resection for Crohn's Disease for modified Rutgeerts' score i2a and i2b categories: Individual patient data meta-analysisYear: 2022
Source: ECCO'22 Virtual
Authors: Pauline Riviere
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundThe modified Rutgeerts' score (mRS) differentiates i2a – lesions confined to the anastomosis – and i2b – neoterminal ileum lesions – categories. Its relevance for therapeutic management of Crohn's disease (CD) patients after ileocolonic resection is still debated. Our objective was to compare the postoperative recurrence (POR) risk in patients with a mRS i2a or i2b score using an individual patient data meta-analysis.
MethodsWe conducted a systematic literature search of Medline, Embase and abstracts from international conferences (until July 2020) to identify all relevant studies reporting the risk of clinical and/or surgical POR and the i2a/i2b status in the year following ileocolonic resection. Initial datasets were obtained from the corresponding authors. Time from endoscopy to clinical and surgical POR was estimated using Kaplan-Meier method. The association between time to event and mRS was evaluated using a mixed Cox with centre as the random effect.
ResultsFrom the 17 studies identified, 7 published between 2008 and 2019 (cohort studies, n=4; clinical trials, n=2) corresponding to a total of 400 patients (median (InterQuartileRange) age at surgery 34 (26,47) years; 52% female) were included. In the year following ileocolonic resection, 189 (47%) patients displayed an i2a mRS and 211 (53%) an i2b. In the i2b group, we observed more male patients (56% versus 41%, p=0.01), more patients with previous ileocolonic resection (31% versus 21%, p=0.03) and temporary ileostomy (14% versus 6%, p=0.03) and an immunosuppressant or antiTNF therapy was more frequently initiated after endoscopy (42% versus 26%, p<0.01 and 36% versus 54%, p<0.01, respectively). The risk of clinical POR at 1, 3 and 5 years was 11% [6%-15%], 25% [18%-32%] and 36% [27%-43%] in the i2a group and 9% [5%-13%], 33% [26%-41%] and 47% [39%-56%] in the i2b group (p=0.63, p=0.12, et p=0.05 respectively). No significant difference was observed in terms of time to clinical POR (Hazard Ratio (HR)=1.27; Confidence Interval 95% [0.91,1.76]; p=0.16) (Figure 1) or surgical POR (HR=0.94; CI95% [0.44,2.00]; p=0.87). After exclusion of patients having initiated an immunosuppressant or a biologic in the 3 months after endoscopy (remaining cohort, n=361), no difference was observed in terms of clinical POR (HR=1.29 [0.92,1.80]; p=0.13) or surgical POR (HR=0.85 [0.39,1.84]; p=0.68).

ConclusionIn this individual patient data meta-analysis, no difference is observed between i2a and i2b mRS subcategories in terms of clinical, surgical or endoscopic POR. Limits of the mRS may explain this lack of predictive value.
Complementary Medicine and Psychotherapy e-CourseYear: 2018
Source: e-Course
Authors: Carolina Palmela, Gianluca Pellino, Sander van der Marel, Kostas Katsanos, Antonio Lopéz-Sanromán
Created: Friday, 28 February 2020, 11:45 AM by Dauren Ramankulov
Last Modified: Wednesday, 2 June 2021, 1:43 PM by ECCO Administrator
This course has been developed for physicians interested in Inflammatory Bowel Disease(s) (IBD). One major aim of this e-learning activity is to increase competence and knowledge with regard to the use of complementary medicines and psychotherapy in IBD patients in order to improve patient outcomes.
After this case you will:
- Know the evidence for the use of dietary supplements, herbs, vitamins, omega-3 fatty acids, probiotics and cannabis in IBD patients
- Be able to identify which herbs or dietary supplements might be useful as complementary therapies in IBD
- Understand the relation between anxiety/depression, stress and fatigue in IBD
- Be able to identify the role of psychological therapies in IBD
- Understand the benefits and side-effects of manipulative and body-based practices
- Counsel your patient on manipulative and body-based practices
Complications associated with anti-TNF therapyYear: 2022
Source: 20th IBD Intensive Course for Trainees
Authors: Yehuda Chowers; Shomron Ben-Horin
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentLearning Objectives - Risks associated with anti-TNF treatments
1. Hypersensitivity reactions
2. Dermatological adverse effects with anti‐TNF therapy
3. Autoimmune‐like disorders
4. Infections and management strategies
5. Malignancy
6. Patients’ selection for anti TNFs vs other biologics
Complimentary treatmentYear: 2021
Source: 15th N-ECCO Network Meeting
Authors: Jost Langhorst
Created: Friday, 1 October 2021, 12:41 PM
Computer-aided endoscopy for scoring histological remission in UCYear: 2022
Source: 7th H-ECCO IBD Masterclass
Authors: Peter Bossuyt
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentEducational objectives:
- To understand the importance of histological remission in IBD
- To understand the hurdles in the assessment of histological remission in IBD
- To understand the potential of automated histological scoring based on artificial analysis
Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease Year: 2014
Source: JCC, Volume 8, Issue 10, 2014
Authors: F.M. Ruemmelea, G. Veres, K.L. Kolho, A. Griffiths, A. Levineg, J.C. Escher, J. Amil Dias, A. Barabinoj, C.P. Braegger, J. Bronskyl, S. Buderus, J. Martín-de-Carpi, L. De Ridder, U.L. Fagerberg, J.P. Hugot, J. Kierkus, S. Kolacekt, S. Koletzko, P. Lionetti, E. Miele, V.M. Navas López, A. Paerregaardy, R.K. Russell, D.E. Serbanaa, R. Shaoul, P. Van Rheenenac, G. Veeremanad, B. Weiss, D. Wilsonaf, A. Dignass, A. Eliakim, H. Winter, D. Turner
Created: Thursday, 30 August 2018, 1:25 PM by Dauren Ramankulov
Children and adolescents with Crohn's disease (CD) present often with a more complicated disease course compared to adult patients. In addition, the potential impact of CD on growth, pubertal and emotional development of patients underlines the need for a specific management strategy of pediatric-onset CD. To develop the first evidenced based and consensus driven guidelines for pediatric-onset CD an expert panel of 33 IBD specialists was formed after an open call within the European Crohn's and Colitis Organisation and the European Society of Pediatric Gastroenterolog, Hepatology and Nutrition. The aim was to base on a thorough review of existing evidence a state of the art guidance on the medical treatment and long term management of children and adolescents with CD, with individualized treatment algorithms based on a benefit-risk analysis according to different clinical scenarios. In children and adolescents who did not have finished their growth, exclusive enteral nutrition (EEN) is the induction therapy of first choice due to its excellent safety profile, preferable over corticosteroids, which are equipotential to induce remission. The majority of patients with pediatric-onset CD require immunomodulator based maintenance therapy. The experts discuss several factors potentially predictive for poor disease outcome (such as severe perianal fistulizing disease, severe stricturing/penetrating disease, severe growth retardation, panenteric disease, persistent severe disease despite adequate induction therapy), which may incite to an anti-TNF-based top down approach. These guidelines are intended to give practical (whenever possible evidence-based) answers to (pediatric) gastroenterologists who take care of children and adolescents with CD; they are not meant to be a rule or legal standard, since many different clinical scenario exist requiring treatment strategies not covered by or different from these guidelines.
Contemporary nutritional approach in adult IBD (Tandem Talk)Year: 2021
Source: 8th P-ECCO Educational Course
Authors: Lihi Godny, Iris Dotan
Created: Friday, 1 October 2021, 12:41 PM
Summary content1. To review current approaches to dietary therapy in adult patients with IBD
2. To discuss the use of dietary therapy to modify IBD
3. To emphasize the role of the dietitian in the multidisciplinary team
Context-dependent roles of High-mobility group box 1 (HMGB1) during intestinal inflammation and carcinogenesisYear: 2022
Source: ECCO'22 Virtual
Authors: Elisabeth Zierz
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundHMGB1 is a ubiquitously expressed nucleoprotein with proinflammatory functions following cellular release. The protein is passively released during tissue necrosis, acting as a damage-associated molecular pattern, but can also be actively secreted by immune cells. Stool and serum HMGB1 levels have been suggested as markers of both inflammatory bowel disease (IBD) activity and colorectal cancer (CRC) invasiveness, and antibody-mediated HMGB1 neutralization was beneficial in animal models of IBD. We explored context-dependent functions of HMGB1 in the injured intestine using novel experimental mice with cell-specific genetic HMGB1 deficiency.
MethodsTo circumvent the postnatal lethality of global HMGB1 deficiency in animals, we used the Cre-lox system to generate enterocyte-specific (Hmgb1ΔIEC, Villin-Cre) and myeloid cell-specific (Hmgb1ΔLysM, LysM-Cre) HMGB1-knockout mice. Animals were subjected to well-established models of acute (DSS, Citrobacter rodentium) and chronic (AOM+DSS, CD45RBhigh T cell transfer colitis, Apc+/min) intestinal injury, followed by clinical, endoscopic, histological and molecular analysis. HMGB1 expression was assessed in human IBD and CRC specimens.
ResultsIBD and CRC biopsies exhibited high levels of HMGB1 expression in epithelia, immune cells, tumor cells and the peritumoral stroma. HMGB1 deficiency from enterocytes and myeloid cells did not alter Citrobacter- or T cell transfer-induced enterocolitis, when epithelial injury was comparably low. In contrast, Hmgb1ΔIEC mice exhibited aggravated DSS-induced colitis, as evidenced by severe weight loss as well as exacerbated neutrophil- and monocyte-driven mucosal inflammation compared to Hmgb1f/f. Whole tissue RNA sequencing indicated defective cellular proliferation in injured Hmgb1ΔIEC intestines. In the AOM+DSS-model, Hmgb1ΔIEC had a comparable tumor burden to Hmgb1f/f, whereas Hmgb1ΔLysM had significantly fewer and smaller tumors, potentially linked to metabolic alterations in the tumor micromilieu. In the Apc+/min model, enterocyte HMGB1 deficiency effectuated more and larger tumors, whereas leukocyte HMGB1 did not affect tumor load.
ConclusionContrasting antibody-mediated HMGB1 neutralization in animal models of IBD, our findings from genetic HMGB1 deletion studies reveal a critical role of enterocyte HMGB1 in the maintenance of the intestinal barrier during severe colitis. Impaired epithelial regeneration or inefficient local immune cell expansion in Hmgb1ΔIEC may account for the aggravated phenotype. HMGB1 from enterocytes and immune cells context-dependently affect maladaptive intestinal would healing, potentially mediated by cell-intrinsic and -extrinsic mechanisms that warrant further investigation.






 Conclusion
Conclusion






