Ulcerative Colitis (UC) Consensus Part 2 - Current management (2012)Year: 2012
Source: JCC, Volume 6, Issue 10, 2012
Authors: Axel Dignass, Rami Eliakim, Fernando Magro, Christian Maaser, Yehuda Chowers, Karel Geboes, Gerassimos Mantzaris, Walter Reinisch, Jean-Frederic Colombel, Severine Vermeire, Simon Travis, James O. Lindsay, Gert Van Assche
Created: Wednesday, 29 August 2018, 4:55 PM by Dauren Ramankulov
Last Modified: Wednesday, 23 January 2019, 5:00 PM by ECCO Administrator
Ulcerative colitis is a lifelong disease arising from an interaction between genetic and environmental factors, observed predominantly in the developed countries of the world. The precise aetiology is unknown and therefore medical therapy to cure the disease is not yet available. Within Europe there is a North–South gradient, but the incidence appears to have increased in Southern and Eastern countries in recent years.1,2 Patients may live with a considerable symptom burden despite medical treatment (66% describe interference with work and 73% with leisure activities3) in the hope that the aetiology of ulcerative colitis will shortly be revealed and a cure emerge. Although this is conceivable in the next decade, clinicians have to advise patients on the basis of information available today. Despite randomised trials there will always be many questions that can only be answered by the exercise of judgement and opinion. This leads to differences in practice between clinicians, which may be brought into sharp relief by differences in emphasis between countries.
Ulcerative Colitis (UC) Consensus Part 3 - Special situations (2012)Year: 2017
Source: JCC: Volume 7, Issue 1, 2017
Authors: Gert Van Assche, Axel Dignass, Bernd Bokemeyer, Silvio Danese, Paolo Gionchetti, Gabriele Moser, Laurent Beaugerie, Fernando Gomollón, Winfried Häuser, Klaus Herrlinger, Bas Oldenburg, Julian Panes, Francisco Portela, Gerhard Rogler, Jürgen Stein, Herbert Tilg, Simon Travis, James O. Lindsay
Created: Wednesday, 29 August 2018, 4:59 PM by Dauren Ramankulov
Last Modified: Friday, 22 February 2019, 9:56 AM by ECCO Administrator
Proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the procedure of choice for most patients with ulcerative colitis (UC) requiring colectomy.1 Pouchitis is a non-specific inflammation of the ileal reservoir and the most common complication of IPAA in patients with UC.2–7 Its frequency is related to the duration of follow up, occurring in up to 50% of patients 10 years after IPAA in large series from major referral centres.1–9 The cumulative incidence of pouchitis in patients with an IPAA for familial adenomatous polyposis is much lower, ranging from 0 to 10%.10–12 Reasons for the higher frequency of pouchitis in UC remain unknown. Whether pouchitis more commonly develops within the first years after IPAA or whether the risk continues to increase with longer follow up remains undefined.
Ulcerative Colitis: From the nurse's perspectiveYear: 2016
Source: ECCO e-Learning
Authors: Karen Kemp, Nienke Ipenburg, Lydia White, and Usha Chauhan
Created: Thursday, 27 February 2020, 4:45 PM by Dauren Ramankulov
This course is designed for nurses with an interest in IBD. The intended results of this activity are increased competence, knowledge and performance and improved patient outcomes.
Upon completion of this activity learners will:
- Have thought through what key information a newly diagnosed patient with Ulcerative Colitis (UC) needs
- Know about the use of mesalazine medication in the treatment of mild to moderate colitis
- Understand issues of compliance for patients on maintenance treatment
- Understand issues of follow-up and support for patients with UC
Ultrasonography in IBD - Audio PodcastYear: 2017
Source: Educational Audio Podcast
Authors: Giovanni Maconi
Created: Friday, 28 February 2020, 11:06 AM by Dauren Ramankulov
Last Modified: Wednesday, 2 June 2021, 4:54 PM by ECCO Administrator
UltrasoundYear: 2018
Source: 5th ECCO-ESGAR Ultrasound-MRI Workshop
Authors: Kucharzik Torsten
Created: Tuesday, 8 May 2018, 11:36 AM
Files: 1
Ultrasound and IBD - e-CourseYear: 2015
Source: e-Course
Authors: Torsten Kucharzik and Fortunata Civitelli
Created: Thursday, 27 February 2020, 4:37 PM by Dauren Ramankulov
Last Modified: Wednesday, 2 June 2021, 12:42 PM by ECCO Administrator
This course is designed for gastroenterologists, paediatricians, surgeons and other interdisciplinary medical experts interested in Inflammatory Bowel Disease(s) (IBD). The intended result of this activity is increased competence and knowledge of the role of imaging techniques, particularly ultrasonography, in the diagnosis and follow-up of IBD patients.
Upon completion of this activity learners will:
- Understand the role of ultrasonography (US) in the initial diagnostic work-up of suspected IBD
- Be able to provide a comprehensive overview of normal and abnormal findings in bowel US, with a focus on IBD
- Be able to understand the usefulness of bowel US in the follow-up of IBD, in assessing disease activity, response to therapy and monitoring disease progression and complications such as stenosis, fistulae and abscesses
Understanding Cancer RiskYear: 2016
Source: Talking Heads
Authors: Ebbe Langholz, Tine Jess
Created: Friday, 22 February 2019, 4:15 PM by ECCO Administrator
Last Modified: Friday, 13 January 2023, 12:13 PM by ECCO Administrator
Unpacking the different popular diets for pediatric Crohn's Disease - concerns around nutritional adequacyYear: 2022
Source: 7th D-ECCO Workshop
Authors: Erin Carmody
Created: Tuesday, 24 May 2022, 8:13 PM
Background
The first line treatment for inducing remission in pediatric Crohn’s disease (CD) is Exclusive Enteral Nutrition (EEN), where a patient drinks a nutritionally complete formula exclusively for 6 to 12 weeks. Despite the effectiveness of EEN, some patients may experience challenges including taste fatigue, monotony, and a lack of social participation with meals. Given these challenges, patients may turn to popular or fad diets for managing their disease. These diets are often restrictive, eliminating a number of foods and exacerbating the risk of underlying nutrient deficiencies in this patient population.
Methods
These case studies involved a nutrient analysis of evidence-based and popular diets for CD, including Crohn’s Disease Exclusion Diet (CDED), CD-TREAT, Specific Carbohydrate Diet (SCD), IBD Anti-inflammatory Diet (IBD-AID), Autoimmune Protocol (AIP) Diet, Gut and Psychology Syndrome (GAPS) Diet, and low FODMAP. Four cases were selected with mild-moderate CD: 11-year-old and 16-year-old, both male and female. A nutrient analysis of sample menus of each diet was completed using Food Processor version of 11.6.0 by ESHA Research. Results were compared to age and gender specific Dietary Reference Intakes (DRIs), population-based dietary intake data, and Health Canada Dietary Guidelines.
Results
Data are presented for Case 1, 11-year-old male. Findings were comparable to other age and gender cases. As compared to Acceptable Macronutrient Distribution Ranges (AMDRs), there was a higher percentage of energy from fats and lower from carbohydrates for the SCD (% kcal, fat and carbohydrate respectively: 59%; 30%), IBD-AID (52%; 37%), AIP Diet (50%; 20%) and GAPS Diet (60%, 21%). Saturated fat intake exceeded recommendations (>10% of energy intake) for CDED (% kcal, 14%) CD Treat (17%), SCD (11%), AIP Diet (15%) and GAPS Diet (20%). Both vitamin D and/or calcium intake were below the Recommended Dietary Allowance (RDA) respectively for CDED (% RDA, vitamin D and calcium respectively: 89%; 86%), SCD (23%; 53%), AIP Diet (14%; 23%), low FODMAP Diet (4%, 96%) and GAPS Diet (calcium, 58%). Adolescent females versus males between the ages of 14-18 years may be at greater risk of inadequate nutrient intake, given the general increase in nutrient requirements yet lower caloric needs.
Conclusion
Given the increase in awareness and interest in popular diets for Crohn’s disease, it is imperative that clinicians are aware of the risks of inadequate nutrient intake with restrictive diets.
Unusual patterns of IBDYear: 2020
Source: 5th H-ECCO IBD Masterclass
Authors: Roger Feakins
Created: Tuesday, 23 June 2020, 5:40 PM
Unusual patterns of IBDYear: 2020
Source: 5th H-ECCO IBD Masterclass
Authors: Roger M. Feakins
Created: Tuesday, 23 June 2020, 4:58 PM
Files: 1
Upadacitinib modulates inflammatory pathways in gut tissue in patients with Ulcerative Colitis: Transcriptomic profiling from the Phase 2b study, U-ACHIEVEYear: 2022
Source: ECCO'22 Virtual
Authors: Bram Verstockt
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundUpadacitinib (UPA), an oral, reversible, Janus kinase (JAK)-1 selective inhibitor can induce clinical and endoscopic remission after 8 weeks in patients (pts) with moderately to severely active Ulcerative Colitis (UC). To provide mechanistic insights into downstream effects of UPA in the intestinal mucosa, we evaluated pharmacodynamic modulation of gene expression in colon biopsies from pts with UC in the Phase 2b study, U-ACHIEVE (NCT02819635). These analyses aimed to link molecular changes to clinical endpoints.
MethodsTranscriptomic data were collected from rectosigmoid biopsies at baseline (BL) and Week (Wk) 8 in a subset of pts in sub-study 1 of U-ACHIEVE (N=88: placebo [PBO], n=15; pooled UPA 15, 30 & 45 mg, n=73). Samples underwent bulk RNA sequencing and differentially expressed genes (DEG) (false discovery rate [FDR]<0.05 & |log fold change [FC]|>1) from BL to Wk 8 were identified with linear mixed-effect models. DEG were analysed with KEGG and GO pathway enrichment and clinical endpoint responder analysis. Cellular profiling with gut cell deconvolution based on defined cell types was undertaken.1
ResultsAt Wk 8, expression of 695 gut genes was modulated (FDR<0.05 & |logFC|>1) from BL after UPA treatment compared with no DEG in PBO pts (including responders). Of these genes, ~70% (n=492) were downregulated and enriched in inflammatory pathways including T- and B-cell effector responses, neutrophil-mediated immunity, and leukocyte chemotaxis. Also, irrespective of directionality, most DEG from BL to Wk 8 in UPA-treated pts were associated with clinical response and remission, and histologic and endoscopic improvement. At Wk 8, deconvoluted cell fractions associated with adaptive but also innate inflammatory cells in the gut of UPA responders were decreased compared with non-responders; in contrast, fractions associated with enterocyte, secretory goblet cell and myofibroblast cells were increased in responder gut tissue (Fig 1). Modulation of genes associated with UC disease activity (OSM & S100A8/9 [calprotectin]), Th1 (TBX21, IFNG), Th2 (GATA3, IL5RA, IL13RA2), Th9 (SPI1), Th17 (IL17A, IL23A, IL21R), B-cell responses (BTK, CD40), barrier function (ESPN, VIL1, CLDN23, OCLN, MUC1/2/12/16/20) and wound repair (ANXA1/6/13, MMP7/9) were associated with clinical improvement at Wk 8 (Fig 2).


ConclusionJAK inhibition with UPA is associated with transcriptional changes in colonic mucosa that are seen with UC disease pathophysiology. Clinical benefit mediated by UPA is associated with modulation of molecular biomarkers of UC disease activity, T-helper-cell differentiation, B-cell-mediated responses, gut barrier function and wound healing.
1. Menden K, et al. Sci Adv 2020;6:eaba2619
Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1Year: 2022
Source: ECCO'22 Virtual
Authors: Séverine Vermeire
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundUpadacitinib (UPA), an oral, reversible JAK inhibitor engineered for increased selectivity for JAK1 over JAK2, JAK3, or tyrosine kinase 2 (TYK2), demonstrated significantly greater efficacy compared with placebo (PBO) for induction of remission in patients with moderately to severely active ulcerative colitis (UC) in two phase 3 trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026). This analysis evaluated the efficacy of UPA on early symptomatic improvement for the first 14 days, using pooled data from U-ACHIEVE Induction and U-ACCOMPLISH.
MethodsU-ACHIEVE and U-ACCOMPLISH were multicentre, double-blind, PBO-controlled trials that enrolled patients who have had moderately to severely UC with an Adapted Mayo Score of 5 to 9 points and centrally reviewed endoscopy subscore of 2 to 3. A total of 998 patients were randomized to receive UPA 45mg once daily (QD) (n=658) or PBO (n=328) for 8 weeks (wks) in a 2:1 ratio. First dose of study drug was administered on Day 0. Improvement in symptoms including stool frequency subscore (SFS), rectal bleeding subscore (RBS), abdominal pain, and bowel urgency were analysed from daily symptom diary data. Multivariate regression analysis was used to determine if early changes in UC symptoms could be used to evaluate a potential correlation with patients’ likelihood of achieving clinical response or clinical remission per Adapted Mayo score at the end of induction.
ResultsBaseline characteristics were similar between both treatment groups. Patients treated with UPA 45 mg QD experienced significant improvement in daily symptoms, with significantly more subjects achieving SFS≤1 (p<0.001), RBS of 0 (p<0.05), and SFS of 0 (p<0.05) as early as day 1 and maintained through day 14 (Figure 1). A significantly higher percentage of patients who received UPA 45 mg QD compared to PBO, achieved abdominal pain=0 and the absence of bowel urgency within 3 days of beginning treatment through day 14 (p<0.05). Multivariate analysis revealed that patients who achieved day 7 SFS≤1 (OR 2.42, 95% CI, 1.53-3.82) were more likely to attain clinical response (Table). Patients who attained day 7 SFS≤1 (OR 2.53, 95% CI 1.59-4.00) or day 7 bowel urgency absent (OR 2.40, 95% CI 1.52-3.79) were more likely to achieve clinical remission at week 8.
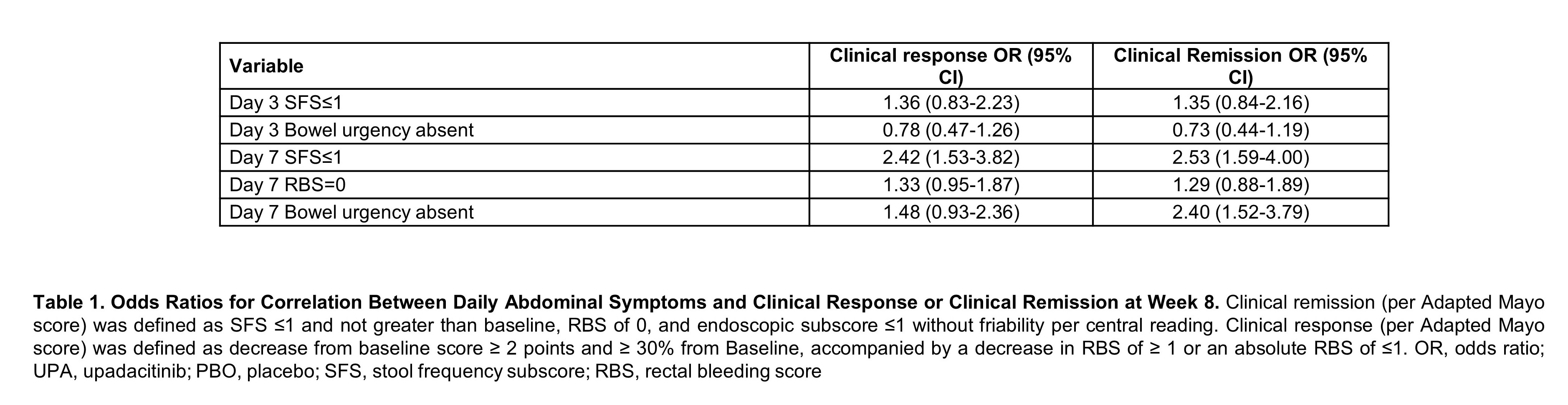
ConclusionUPA 45 mg QD significantly improved UC symptoms as early as day 1, providing patients with rapid symptom relief. Patients who achieved early symptom improvement were more likely to attain clinical remission or clinical response at week 8. [Clinicaltrials.gov, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026)]
Update on the RICCO studyYear: 2017
Source: 6th S-ECCO IBD Masterclass
Authors: Maggiori L.
Ileo caecal resection, Post operative complications, Post operative medical management, Anti TNF agents
Files: 1



