Exabis Library
Welcome to the e-CCO Library!
Efficacy and safety of extended induction treatment with upadacitinib 45 mg once daily followed by maintenance upadacitinib 15 or 30 mg once daily in patients with moderately to severely active Ulcerative Colitis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy and safety of filgotinib for the treatment of perianal fistulizing Crohn’s Disease: Results from the phase 2 DIVERGENCE 2 study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy and safety of filgotinib in patients with Ulcerative Colitis stratified by age: Post hoc analysis of the phase 2b/3 SELECTION and SELECTIONLTE studies
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
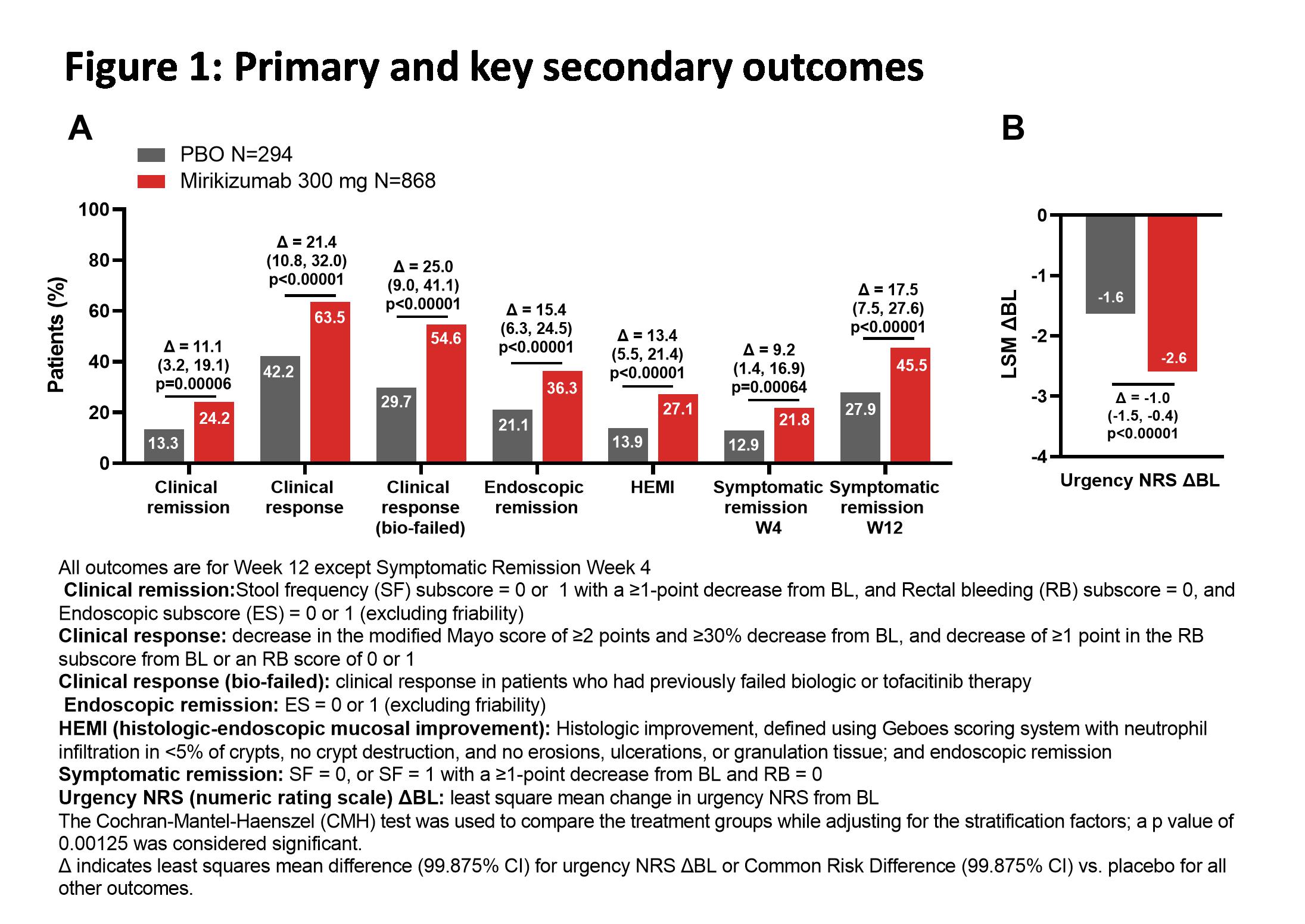
Efficacy and safety of mirikizumab as induction therapy in patients with moderately to severely active Ulcerative Colitis: Results from the Phase 3 LUCENT-1 study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy and safety of vedolizumab in patients with chronic active pouchitis refractory to anti-TNF therapy: Results of a retrospective multicenter study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of biologic drugs in short-duration versus long-duration Inflammatory Bowel Disease: A systematic review and an individual-patient data meta-analysis of randomized controlled trials
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of risankizumab induction and maintenance therapy by baseline Crohn’s Disease location: Post hoc analysis of the phase 3 ADVANCE, MOTIVATE, and FORTIFY studies
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of risankizumab rescue therapy in patients with moderately to severely active Crohn’s Disease and inadequate response to risankizumab maintenance therapy
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Efficacy of the treat-to-target approach in modifying disease course with ustekinumab in patients with moderate-to-severe Crohn’s Disease: Results from the STARDUST trial
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Elastography and CEUS to characterize strictures and abdominal masses in Crohn’s Disease
2019
6th ECCO-ESGAR Ultrasound Workshop - Advanced
Wednesday, 5 June 2019, 9:01 PM
Endoscopic outcomes and therapeutic drug monitoring with Vedolizumab: the Leuven experience
2019
JCC Podcast
Wednesday, 9 October 2019, 2:04 PM by Dauren Ramankulov
Tuesday, 13 October 2020, 3:49 PM by Dauren Ramankulov
Endoscopy or ultrasound for disease activity assessment? (Tandem Talk)
2021
ECCO'21 Virtual
Friday, 1 October 2021, 12:41 PM
Entero MRI - what to look for
2021
3rd ECCO Basic Imaging Workshop in collaboration with ESGAR: Ultrasound and MRI
Friday, 1 October 2021, 12:41 PM
Environmental factors as therapy (diet modification, EEN feeding)
2017
ECCO'17 Barcelona
Wednesday, 15 March 2017, 1:51 PM by ECCO Administrator
1
ESPGHAN-ECCO Guidelines: Update on Paediatric UC Treatment
2018
ECCO'18 Vienna
Friday, 23 March 2018, 12:23 PM
1
Evaluating segmental healing with the modified Mayo endoscopic score (MMES) has a clear additional value in predicting long-term outcome in patients with Ulcerative Colitis: Results from a prospective cohort study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM


















.png)
.png)