Exabis Library
Welcome to the e-CCO Library!
Identification and characterization of T-cell receptor sequences associated with Crohn’s Disease
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Identification and development of a 1st in class naturally-derived protein that drives mucosal healing and is orally delivered by an engineered cellular therapy targeting the gastro-intestinal tract
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Identification of an intestine-derived ex-Trm population in the blood of healthy individuals and patients with Inflammatory Bowel Disease
2022
8th Y-ECCO Basic Science Workshop
Tuesday, 24 May 2022, 8:13 PM
Identifying Poor Prognosis in CD
2019
Talking Heads
Friday, 22 February 2019, 3:12 PM by ECCO Administrator
Wednesday, 2 June 2021, 11:59 AM by ECCO Administrator
IgG4 pathology – background and its relevance to IBD
2020
5th H-ECCO IBD Masterclass
Tuesday, 23 June 2020, 5:40 PM
Imaging Modalities in IBD
2016
IBD Blue Book - ECCO e-Learning
Friday, 28 February 2020, 10:54 AM by Dauren Ramankulov
Friday, 28 February 2020, 10:55 AM by Dauren Ramankulov
Immune cell migration in the control of organ-specific disease manifestation
2020
8th SciCom Workshop
Tuesday, 23 June 2020, 5:40 PM
Immunological effects of nutritional interventions in IBD
2019
4th D-ECCO Workshop
Wednesday, 5 June 2019, 9:01 PM
Impact of corticosteroid usage on efficacy and safety outcomes in patients receiving upadacitinib for Ulcerative Colitis
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Impact of COVID-19 on clinical research in IBD
2022
8th ClinCom Workshop
Tuesday, 24 May 2022, 8:13 PM
In-depth characterisation of the serum antibody epitope repertoire in Inflammatory Bowel Disease by high-throughput phage-displayed immunoprecipitation sequencing
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
In-depth characterisation of the serum antibody epitope repertoire in Inflammatory Bowel Disease by high-throughput phage-displayed immunoprecipitation sequencing
2022
8th Y-ECCO Basic Science Workshop
Tuesday, 24 May 2022, 8:13 PM
Increasing incidence of Inflammatory Bowel Disease in a high prevalence country: A nationwide study in Finland
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Increasing incidence of pouchitis among patients undergoing ileal pouch-anal anastomosis between 1996 and 2018: A population-based Danish cohort study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Infections with biologics and small molecules: Impact on drug positioning
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Inflammatory Bowel Disease (IBD) and Solid Organ Transplantation. Natural history of pre-existing and de novo IBD patients. (EITOS study of GETECCU)
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Inflammatory pathways
2017
15th IBD Intensive Advanced Course
Wednesday, 15 March 2017, 1:13 PM by Vesna Babaja
1
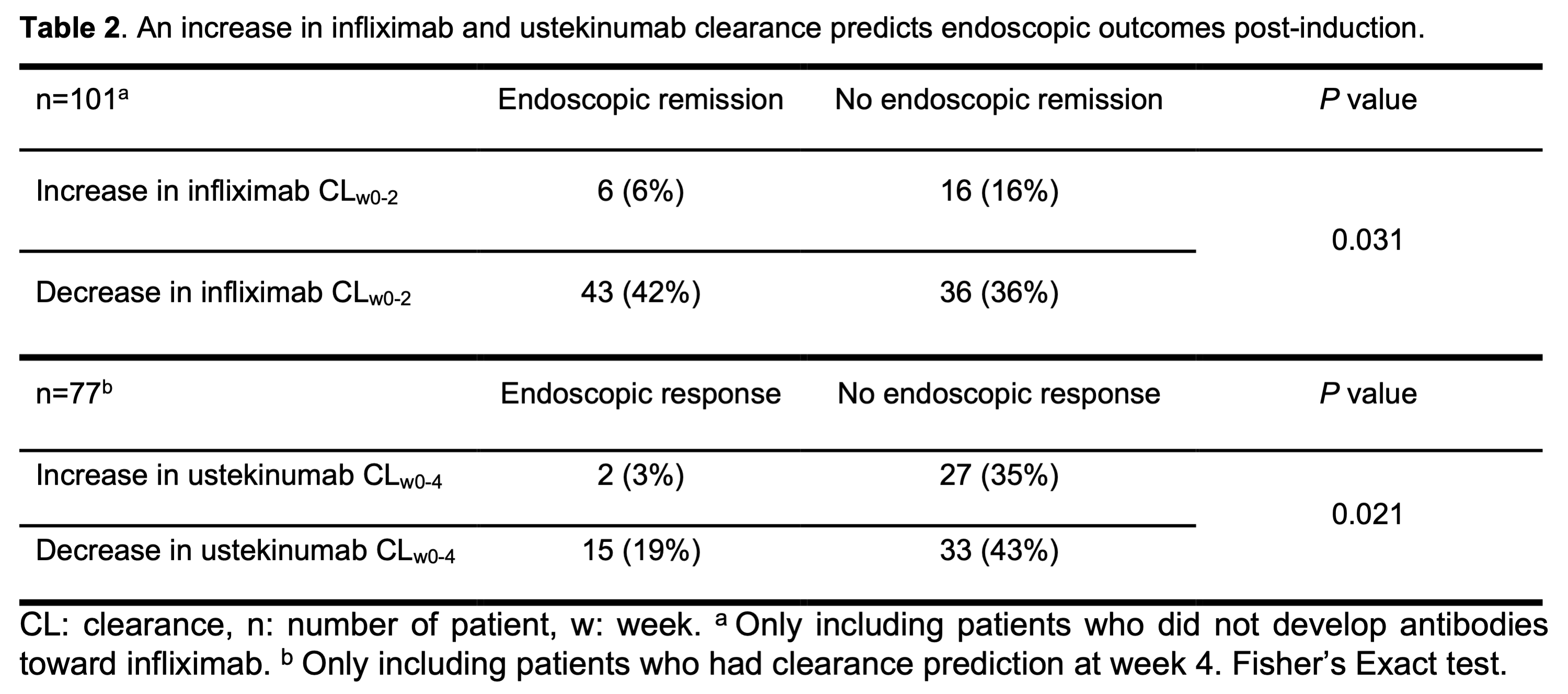
Infliximab and ustekinumab clearance during induction predicts post-induction endoscopic outcomes in patients with Crohn’s Disease
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Initial work up after diagnosis of IBD (Tandem talk)
2022
6th Basic ECCO: EduCational COurse for Industry
Tuesday, 24 May 2022, 8:13 PM





 Conclusion
Conclusion