Exabis Library
Welcome to the e-CCO Library!
Challenges and opportunities of virtual clinics in IBD
2022
16th N-ECCO Network Meeting
Tuesday, 24 May 2022, 8:13 PM
Chronic abdominal pain in IBD patients in remission: Real-world data on contributing factors
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Classical pharmacovigilance: Still useful?
2021
4th School for Clinical Trialists
Friday, 1 October 2021, 12:41 PM
Classifying perianal fistulising Crohn’s Disease: An expert-consensus to guide decision-making in daily practice and clinical trials
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Clinical efficacy and safety of guselkumab maintenance therapy in patients with moderately to severely active Crohn’s Disease: Week 48 analyses from the phase 2 GALAXI 1 study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Clinical outcomes of COVID-19 and Impact on Disease Course in Patients with Inflammatory Bowel Disease
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Clinical trials: Lessons from the past
2021
5th Advanced ECCO: EduCational COurse for Industry
Friday, 1 October 2021, 12:41 PM
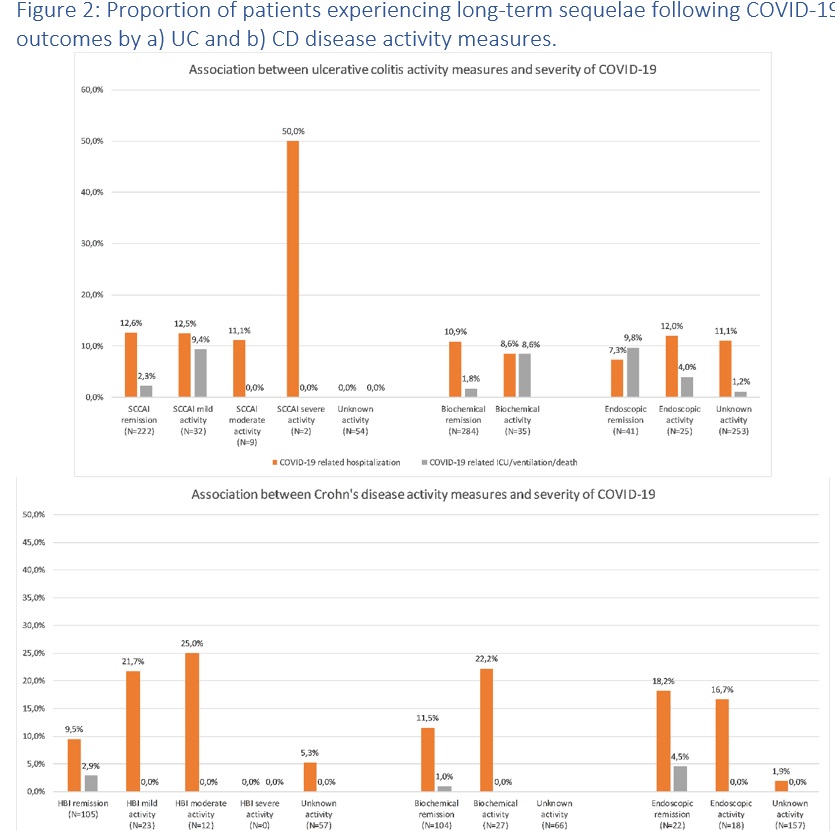
Clinical, biochemical and endoscopic disease activity of Inflammatory Bowel Diseases are not associated with the severity or long-term outcomes of COVID-19 – A Danish prospective population-based cohort study
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Closing remarks, N-ECCO in 2018 and beyond
2018
12th N-ECCO Network Meeting
Friday, 23 March 2018, 12:23 PM
1
Colonic versus small bowel CD: Same mechanism - same treatment?
2020
ECCO'20 Vienna
Tuesday, 23 June 2020, 5:40 PM
Combined approach for intestinal sparing in CD?
2017
ECCO'17 Barcelona
Wednesday, 15 March 2017, 2:50 PM by ECCO Administrator
1
Common and distinct pathways in intestinal and non-intestinal immune mediated inflammatory disorders
2020
8th SciCom Workshop
Tuesday, 23 June 2020, 5:40 PM
Communicating risks – IBD research nurse and physician perspective (Tandem Talk)
2021
4th School for Clinical Trialists
Friday, 1 October 2021, 12:41 PM








 Conclusion
Conclusion

 Conclusion
Conclusion