Sexual Dysfunction in IBD Year: 2018
Source: 12th N-ECCO Network Meeting
Authors: Katsanos Konstantinos
Created: Friday, 23 March 2018, 12:23 PM
Last Modified: Wednesday, 26 May 2021, 11:16 AM by ECCO Administrator
Files: 1
Short bowelYear: 2022
Source: 11th S-ECCO IBD Masterclass
Authors: Carolynne Vaizey
Created: Tuesday, 24 May 2022, 8:13 PM
Shorter disease duration is associated with better outcomes in patients with moderately to severely active Crohn’s Disease treated with risankizumab: Results from the phase 3 ADVANCE, MOTIVATE, and FORTIFY studiesYear: 2022
Source: ECCO'22 Virtual
Authors: Laurent Peyrin-Biroulet
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundAn association between shorter disease duration and improved clinical efficacy has been shown in post hoc analyses of clinical trial data with biological therapies in Crohn’s disease (CD). The efficacy and safety of risankizumab (RZB) as induction and maintenance therapy have been recently reported. Here, the efficacy of RZB stratified by baseline CD duration is reported.
MethodsIn ADVANCE (NCT03105128) and MOTIVATE (NCT03104413), patients with moderately to severely active CD received intravenous (IV) RZB induction therapy or placebo (PBO) for 12 weeks. Patients with clinical response to RZB IV induction were re-randomised in a 52-week maintenance study (FORTIFY, NCT03105102) to receive subcutaneous (SC) RZB or PBO (ie, withdrawal). For this post-hoc analysis, patient subgroups were stratified by years of CD duration at baseline (< 2, 2–5, > 5–10, and > 10 years). Induction analyses focused on patients who received RZB 600 mg IV or PBO for 12 weeks. As all patients who entered maintenance responded to RZB IV induction, maintenance analyses were limited to those patients who responded to induction and then received RZB 360 mg SC for 52 weeks. Clinical and endoscopic outcomes were evaluated using nonresponder imputation incorporating multiple imputation to handle missing data due to impact of the COVID-19 pandemic. Safety was assessed throughout the studies.
ResultsThe induction and maintenance analyses included 527 patients who received RZB 600 mg IV and 141 patients who received RZB 360 mg SC, respectively. At the end of induction (week 12), patients with CD duration of < 2 years achieved higher rates of endoscopic outcomes with IV RZB induction vs patients with longer durations of disease (Figure 1), and regardless of baseline CD duration, greater proportions of RZB-treated patients achieved clinical remission (defined by stool frequency and abdominal pain), endoscopic response, endoscopic remission, and ulcer-free endoscopy vs PBO (P ≤ .05). Clinical remission rates at week 12 were numerically higher in patients with CD duration of < 5 years vs > 5 years (Figure 1). Similar results for improved clinical and endoscopic outcomes associated with shorter disease duration were observed at week 52 with RZB 360 mg SC maintenance treatment (Figure 2). RZB was well tolerated with lower rates of serious adverse events and serious infections vs PBO in induction, across CD duration subgroups.


ConclusionRZB induction and maintenance therapy was effective and well tolerated with a safety profile generally similar across CD duration subgroups. Achievement of clinical and endoscopic endpoints were higher in patients with shorter duration of CD, suggesting that earlier introduction of RZB therapy may lead to improved outcomes.
Single-cell analysis of gut mucosal- and peripheral blood cells in ulcerative colitis patients undergoing vedolizumab treatmentYear: 2022
Source: 8th Y-ECCO Basic Science Workshop
Authors: Naomi Karmi
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundVedolizumab (VDZ), a monoclonal antibody that targets α4β7 integrin, was approved to treat moderate-to-severe ulcerative colitis (UC) based on the presumption that it blocks T cell recruitment to the inflamed intestinal mucosa. The clinical evidence suggests that up to 50% of UC patients do not achieve disease remission under VDZ treatment. This study aims to identify changes in cell abundances and molecular pathways associated with VDZ response in UC. To this end, we included anti-tumor necrosis factor (anti-TNF)-naïve and anti-TNF-exposed patients with active UC, and utilized single-cell RNA sequencing (scRNAseq) and high-dimensional flow cytometry (Cytek) to assess the peripheral blood and the gut mucosal compartments.
MethodsGut mucosal biopsies from inflamed and non-inflamed regions, and peripheral blood mononuclear cells (PBMCs) were obtained from UC patients 2 wks before (t0) and 14 wks after (t4) the start of VDZ administration. Response to treatment was prospectively evaluated based on endoscopic assessment (defined as a decrease in total Mayo score between t0 and t4) and physician global assessment (PGA) that incorporates disease activity score and biochemical measurements.
ResultsA total of 25 UC patients (pts) were included: 44% anti-TNF-naïve. Endoscopic response to VDZ was observed in 32% of UC pts, while 56% of pts showed response based on PGA. The VDZ response rate (by PGA) was higher in anti-TNF-naïve pts vs anti-TNF-exposed pts (82% vs 36% responders, respectively). A preliminary analysis was performed on samples from 8 (out of 25) UC pts, profiling >70,000 gut mucosal cells and >25,000 PBMCs. Within the mucosal compartment, at t0 we identified immune cells (50% of all captured cells), stromal cells (10%), and epithelial cells (40%). Upon inflammation, the proportion of immune cells increased to 70%, stromal cells to 20%, while epithelial cells depleted to 10%. Notably, all main identified immune cell lineages – T cells, B cells and myeloid cells – contributed to the expansion of the immune cell compartment in inflamed mucosa. In line with scRNAseq data, we identified all major immune cell populations and detected expression of both the classic gut-directed and the redundant trafficking integrins by Cytek.
ConclusionThe preliminary results substantiate our current understanding of VDZ biology in UC. We confirm that anti-TNF-naïve pts have a higher response rate to VDZ vs anti-TNF-exposed pts. With this unique cohort, our study has the power to further explore molecular mechanisms and pathways that underlie VDZ response at the single-cell level.
Skin cancer in IBD: Practical advice from a dermatologistYear: 2022
Source: ECCO'22 Virtual
Authors: An Van Laethem
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentNon melanoma skin cancer (NMSC) is amongst the most common cancers worldwide and the incidence of both melanoma and NMSC is still rising. This is not only due to people reaching an older age, but also to the increasing amount of people receiving immunosuppressive medication. While immunosuppression in organ transplant patients serves as the most well-known model for these iatrogenic induced skin cancers, alternative forms of immunomodulating therapies, such as the biologicals, also caught attention because of their potential to disrupt skin cancer immunosurveillance. Data on biologicals, with anti-TNFα most studied, are more controversial, however, and diverge according to the inflammatory disease (Inflammatory Bowel Disease (IBD), psoriasis or rheumatoid arthritis) for which they are given.
In IBD patients there is an elevated risk for both NMSC as well as melanoma. Whereas NMSC in IBD patients seems associated with current or past use of thiopurines, it is still unclear if the higher risk for melanoma in IBD patients can be attributed to the use of TNF inhibitors. As the cutaneous malignancies in these immunosuppressed patients behave often more aggressively, preventive strategies are mainstay in the approach of skin care, requiring good coordination between the gastroenterologist and the dermatologist. For IBD patients with a present or past history of a cutaneous malignancy, a multidisciplinary care involving the gastroenterologist and dermatologic or oncologic specialties will have to guarantee the balance between the IBD treatment and the management of the malignancy when facing challenges as maintaining local tumour control, avoiding cancer recurrence/new cancer with future IBD treatment or dealing with checkpoint-inhibitor colitis during management of advanced or metastatic skin cancer.
Slide seminarYear: 2021
Source: 6th H-ECCO IBD Masterclass
Authors: Pamela Baldin
Created: Friday, 1 October 2021, 12:41 PM
Summary contentIn this slide seminar a complicated case of IBD in a pediatric patient will be discussed.
Educational objective:
- evaluation of a case in relation of clinical information
- to think on different diagnosis in IBD
Slide seminarYear: 2021
Source: 6th H-ECCO IBD Masterclass
Authors: Ann Driessen
Created: Friday, 1 October 2021, 12:41 PM
Summary contentObjectives
1. The role of endoscopy in the surveillance of IBD-patients
2. The diagnosis of dysplasia and its subtypes on biopsies
3. The consequences for the treatment of the patient
Due to the continuous inflamed state of the mucosa, ulcerative colitis and Crohn’s disease patients are at risk of developing colorectal cancer at an earlier age and with a poorer prognosis. Hence continuous endoscopic surveillance with sampling of biopsies is necessary to detect the preneoplastic lesions in an early stage. The SCENIC classification is a new endoscopic classification, which categorizes the lesions in to invisible and visible dysplasia.. Histologically these lesions consist of different subtypes, of which the adenomatous type is the most common. The presence of an inflamed mucosa complicates its diagnosis, resulting in a high interobserver variability in the categories indefinite for dysplasia and low grade dysplasia. Hence the ECCO-guidelines recommend to confirm the diagnosis of dysplasia by an expert pathologist in gastrointestinal pathology.
Slide seminarYear: 2021
Source: 6th H-ECCO IBD Masterclass
Authors: Gert De Hertogh
Created: Friday, 1 October 2021, 12:41 PM
Summary contentEductional objective: To illustrate a rare mimicker of IBD pathology.
A middle-aged woman of Turkish origin complained of abdominal pain, watery diarrhea and vomiting since 3 weeks.
Lab tests were negative. Colonoscopy showed moderate to severe, patchy pancolitis.
Pathology was unclear.
She was treated with antibiotics and painkillers.
She went first into remission, and then did a relapse after one month.
At this time there was also arhtritis and oral as well as genital sores.
Colonoscopy showed patchy ulceration with dense perivascular inflammatory cell infiltrates, but without granulomas.
Your diagnosis?
Slide seminar - Macroscopic pathology of IBDYear: 2021
Source: 6th H-ECCO IBD Masterclass
Authors: Francesca Rosini
Created: Friday, 1 October 2021, 12:41 PM
Summary contentUlcerative Colitis and Crohn's disease have different macroscopic appearances.
Both diseases show unique and peculiar macroscopic features and it is important to recognise them in order to sample IBD specimens correctly.
The macroscopic examination is the first step for a correct pathological analysis and it is essential for the histological examination.
Educational objectives:
-To identify basic macroscopic features of UC and CD.
-To sample the specimens correctly.
-To recognise elementary lesions.
-To do not underestimate IBD samples.
Small bowel dysplasia/malignancy: Proactice or reactive?Year: 2022
Source: ECCO'22 Virtual
Authors: Pascal Juillerat
Created: Tuesday, 24 May 2022, 8:13 PM
Summary content1. To learn about the epidemiology of Small Bowel Adenocarcinoma.
2. To understand the absolute risk and relative risk linked to it.
3. To review practical management of Dysplasia/ SBA
Small molecules are backYear: 2018
Source: ECCO'18 Vienna
Authors: Peyrin-Biroulet Laurent
Created: Friday, 23 March 2018, 12:23 PM
Files: 1
Somatic mutations and their therapeutic implications in IBDYear: 2022
Source: 10th SciCom Workshop
Authors: Timothy Raine
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentEducational objectives
To discuss the evidence for somatic mutation in the IBD affected colonic epithelium
To consider mechanisms mutagenesis
To discuss evidence for selection pressures on mutations and their implications for cancer risk and inflammation
Standardised histological report in IBDYear: 2022
Source: 7th H-ECCO IBD Masterclass
Authors: Roger M. Feakins
Created: Tuesday, 24 May 2022, 8:13 PM
Summary content1. To identify the areas where standardisation of IBD pathology reporting is achievable and the areas requiring improvement.
2. To recognise the importance of a multidiscipinary approach and good communication.
3. To consider the development of a consistent approach to the assessment and description of IBD histology.
4. To be aware of the diversity of approaches to the assessment of histological acitvity.
4. To explore the ideal ways in which to construct the summary and conclusion of an IBD pathology report.
5. To be aware of the existence and value of guidelines and datasets in pathology generally and in IBD pathology in particular.
Standardized faecal microbiota transplantation with microbiome-guided donor selection in active UC patients: A randomized, placebo-controlled intervention studyYear: 2022
Source: ECCO'22 Virtual
Authors: Clara Caenepeel
Created: Tuesday, 24 May 2022, 8:13 PM
BackgroundFour randomized controlled trials studying faecal microbiota transplantation (FMT) in active UC patients showed variable success rates. The efficacy of FMT appears to be influenced by various factors including donor- and procedure-specific characteristics. We hypothesized that the outcome of FMT in patients with active UC could be improved by donor preselection on microbiota level, by using a strict anaerobic approach, and by repeated FMT administration.
MethodsThe RESTORE-UC trial (NCT03110289) was a national, multi-centric double-blind, sham-controlled randomized trial. Active UC patients (Total Mayo score 4-10 with endoscopic sub-score ≥2) were randomly allocated (1:1) to receive 4 anaerobic-prepared superdonor (S) FMT or autologous (A) FMT (Figure 1) by permutated blocks (2 and 4) and stratified for weight, concomitant steroid use, and therapy refractoriness.
S-FMTs were selected after a rigorous screening excluding samples with Bacteroides2 enterotype, high abundances of Fusobacterium, Escherichia coli and Veillonella and the lowest microbial loads (Q1).
A futility analysis after 66% (n=72) of inclusions was planned per protocol including a modified intention-to-treat (mITT) analysis using non-responder imputation (NRI) for patients receiving at least one FMT. The primary endpoint was steroid-free clinical remission (Total Mayo ≤ 2, with no sub-score >1) at week 8.
ResultsBetween March 2017-2021, 72 patients signed the ICF and 66 were randomly allocated to S-FMT (n=30) or A-FMT (n=36) and received at least one FMT. In the S-FMT and the A-FMT resp. 4 and 5 patients terminated the trial early due to worsening of colitis (4 in both arms) or FMT enema intolerance (1 A-FMT). They were included in the mITT analysis using NRI (Fig. 2). Both study arms were matched for baseline characteristics (Table 1), yet a trend (p= 0.066) towards higher concomitant biological use in the S-FMT arm was observed.
After 66% of intended inclusions, the primary endpoint was reached in 3/30 (10%) S-FMT and 5/31 (13.9%) patients randomized to A-FMT (p=0.72).
As the predefined minimum difference between both treatment arms was not attained, the study was stopped due to futility. The full set of endpoints are summarized in Table 2.Of note, no patients on concomitant biologicals reached the primary endpoint.
There were 2 serious adverse events in the A-FMT arm: dysuria requiring hospitalization and worsening of UC requiring colectomy.


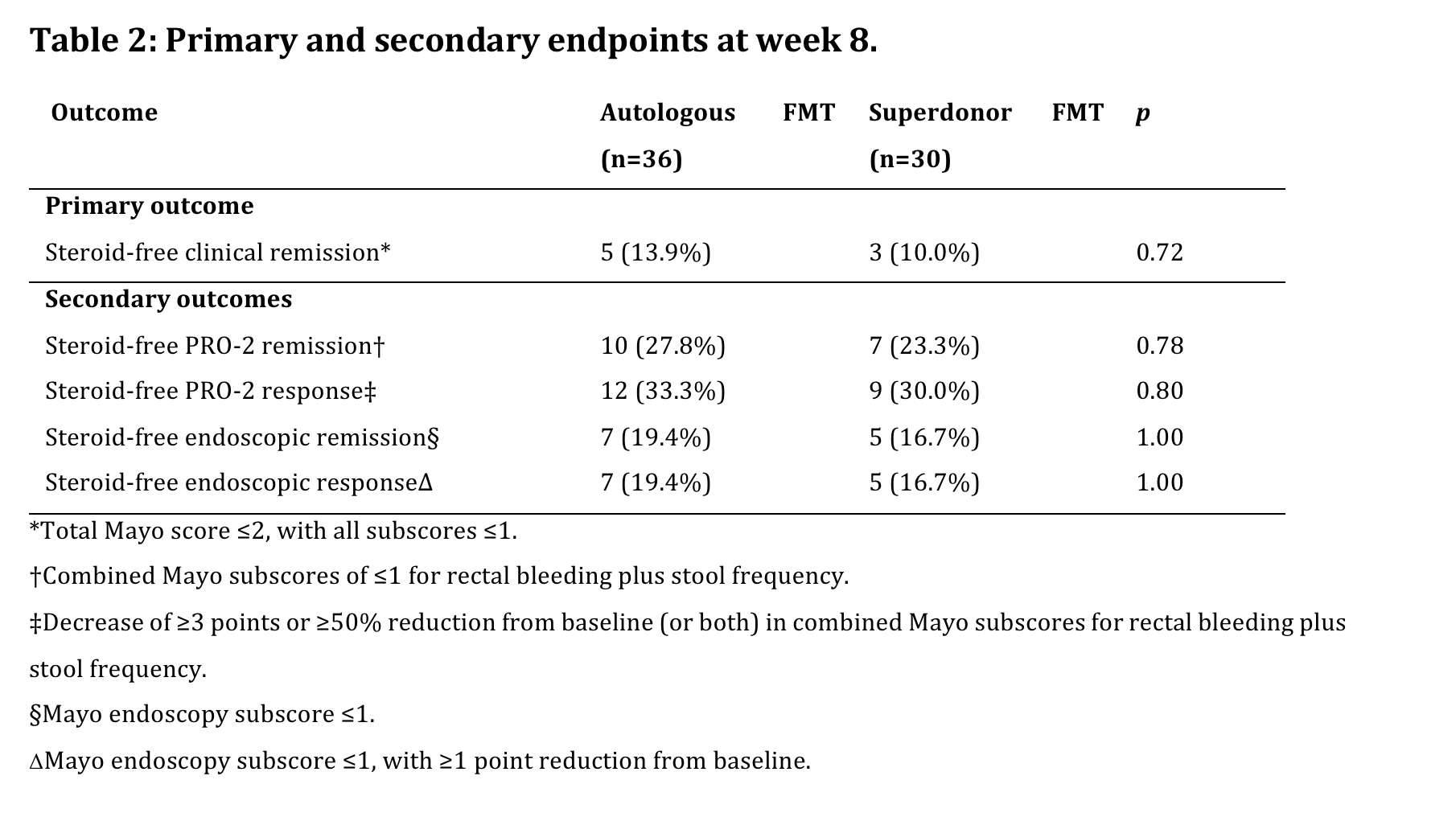
ConclusionIn this double-blind sham-controlled trial comparing repeated administrations of anaerobic-prepared S-FMT with A-FMT in patients with active UC, no significant difference in steroid-free remission rates at week 8 were observed. The FMT procedure was generally well tolerated, and no new safety signals were observed.
Standardized reporting and documentationYear: 2022
Source: 9th ECCO Ultrasound Workshop - Advanced in collaboration with ESGAR
Authors: Torsten Kucharzik
Created: Tuesday, 24 May 2022, 8:13 PM
Summary contentOptimization and standardization of imaging reporting is currently an unmet need and would facilitate the comparison between different reports and communication between the different specialties involved in IBD. The current presentation summarizes results of a consensus guideline that has been developed by members of ECCO, IBUS and ESGAR. The consensus group identified standardized parameters and suggests how to report and how to characterize findings of cross-sectional imaging that encompasses MRI, CT, IUS, endoanal ultrasonography [EAUS] and transperineal ultrasonography [PUS] in IBD. These methods are used for diagnosis, assessment of disease activity and severity, and to detect complications and monitor disease course. Mural and extramural disease manifestations beyond the reach of the endoscope can be visualized and determined by cross sectional imaging.
The core elements of the presentation will describe the imaging parameters of assessment of disease activity and severity as well as intra- and extramural complications of IBD in a standardized manner. The presentation will suggest vital data for each reporting type, and proposes possible strategies to optimize and standardize reporting quality of cross-sectional imaging in IBD. Similarities and differences in reporting between MRI/CT and IUS will be identified and addressed. Practical examples will be provided on how to standardize reporting of individual cases in daily clinical practice.
SteroidsYear: 2017
Source: 15th IBD Intensive Advanced Course
Authors: Vavricka S.
Last Modified: Monday, 10 May 2021, 11:41 AM by ECCO Administrator
Budesonide, Corticosteroids
Files: 1





