Exabis Library
Welcome to the e-CCO Library!
Surgical intervention in UC: When, how and what afterwards (Tandem talk)
2022
6th Basic ECCO: EduCational COurse for Industry
Tuesday, 24 May 2022, 8:13 PM
Surgical Trends in the Biologic Era
2019
Educational Audio Podcasts
Friday, 28 February 2020, 3:57 PM by Dauren Ramankulov
Friday, 13 January 2023, 11:41 AM by ECCO Administrator
Talking Heads: Communicating Risk to Patients
2014
Talking Heads
Monday, 17 August 2020, 10:42 AM by Dauren Ramankulov
Talking Heads: Fecal Transplantation
2014
Talking Heads
Monday, 17 August 2020, 10:43 AM by Dauren Ramankulov
Talking Heads: Nutritional Therapy
2014
Talking Heads
Monday, 17 August 2020, 10:44 AM by Dauren Ramankulov
Talking Heads: Travelling with IBD
2015
Talking Heads
Friday, 13 January 2023, 11:54 AM by ECCO Administrator
Tandem talk: The future IBD clinic - the role of dietitian and nurse in management of IBD patients
2019
4th D-ECCO Workshop
Wednesday, 5 June 2019, 9:01 PM
Tandem Talk: When IUS, when MRI in daily IBD practice?
2021
3rd ECCO Basic Imaging Workshop in collaboration with ESGAR: Ultrasound and MRI
Friday, 1 October 2021, 12:41 PM
The Arborisation index: An MRI-based measure of mesenteric hyperaemia in Crohn’s Disease
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
The association of anti-tumor necrosis factor and thiopurine therapy with the risk of lymphoma among Inflammatory Bowel Disease patients: A nation-wide study from the epi-IIRN
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
The breastmilk proteomics of women with Inflammatory Bowel Disease (IBD) and its impact on fecal calprotectin and microbiota composition in their babies
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
The contribution of ultrasound in Ulcerative Colitis
2019
6th ECCO-ESGAR Ultrasound Workshop - Advanced
Wednesday, 5 June 2019, 9:01 PM
The COSTA study: COlonic Salvage by Therapeutic Appendectomy
2021
10th S-ECCO IBD Masterclass
Friday, 1 October 2021, 12:41 PM
The effects of maintenance therapy with upadacitinib on abdominal pain, bowel urgency, and fatigue in patients with moderately to severely active Ulcerative Colitis: Phase 3 U-ACHIEVE maintenance results
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
The efficacy and safety of guselkumab induction therapy in patients with moderately to severely active Ulcerative Colitis: Phase 2b QUASAR Study results through week 12
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM









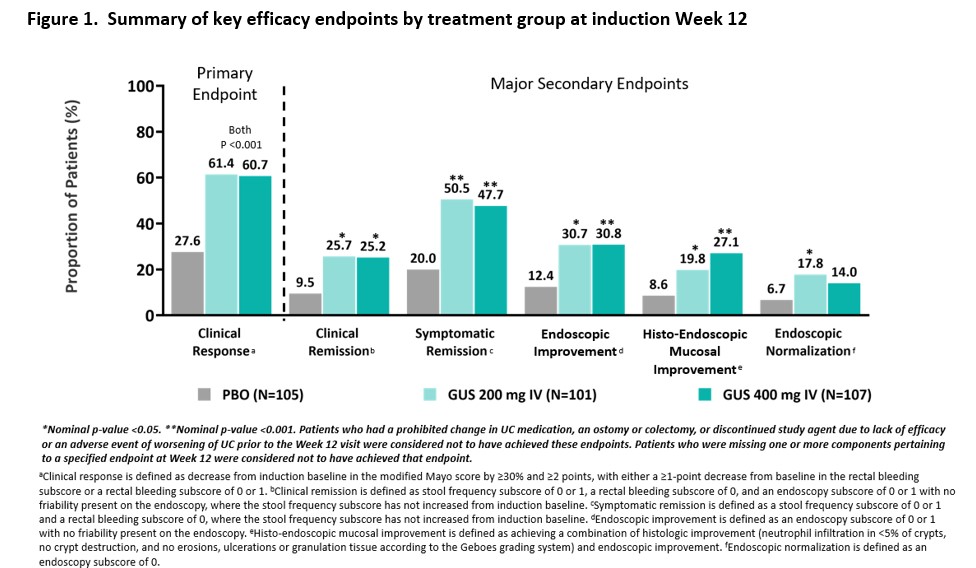
 Conclusion
Conclusion