Exabis Library
Welcome to the e-CCO Library!
Type of patient education impacts the willingness to switch from an IV to SC of a biological in patients with Inflammatory Bowel Disease: a multicentre, comparative study.
2022
16th N-ECCO Network Meeting
Tuesday, 24 May 2022, 8:13 PM
UC cases presentation A) Newly diagnosed UC B) Persistent active UC C) Panel discussion - Q&A and conclusion
2022
2nd ECCO Postgraduate Course in IBD
Tuesday, 24 May 2022, 8:13 PM
UC cases presentation: A) New diagnosed UC B) Persistent active UC
2021
1st ECCO Postgraduate Course in IBD
Friday, 1 October 2021, 12:41 PM
UC Management
2020
11th N-ECCO School
Tuesday, 23 June 2020, 5:40 PM
Thursday, 17 June 2021, 4:02 PM by ECCO Administrator
Ulcerative Colitis or Crohn’s Disease? The pathologist’s contribution
2020
5th H-ECCO IBD Masterclass
Tuesday, 23 June 2020, 5:40 PM
Ultrasonography in IBD - Audio Podcast
2017
Educational Audio Podcast
Friday, 28 February 2020, 11:06 AM by Dauren Ramankulov
Wednesday, 2 June 2021, 4:54 PM by ECCO Administrator
Under what circumstances can Faecal Calprotectin replace colonoscopy
2016
Talking Heads
Friday, 22 February 2019, 4:23 PM by ECCO Administrator
Wednesday, 2 June 2021, 11:14 AM by ECCO Administrator
Understanding Cancer Risk
2016
Talking Heads
Friday, 22 February 2019, 4:15 PM by ECCO Administrator
Friday, 13 January 2023, 12:13 PM by ECCO Administrator
Understanding the mechanisms of anti-TNF treatment failure in patients with Crohn’s Disease: A proteomic analysis of the PANTS cohort
2022
8th Y-ECCO Basic Science Workshop
Tuesday, 24 May 2022, 8:13 PM
Unpacking the different popular diets for pediatric Crohn's Disease - concerns around nutritional adequacy
2022
7th D-ECCO Workshop
Tuesday, 24 May 2022, 8:13 PM
Upadacitinib modulates inflammatory pathways in gut tissue in patients with Ulcerative Colitis: Transcriptomic profiling from the Phase 2b study, U-ACHIEVE
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
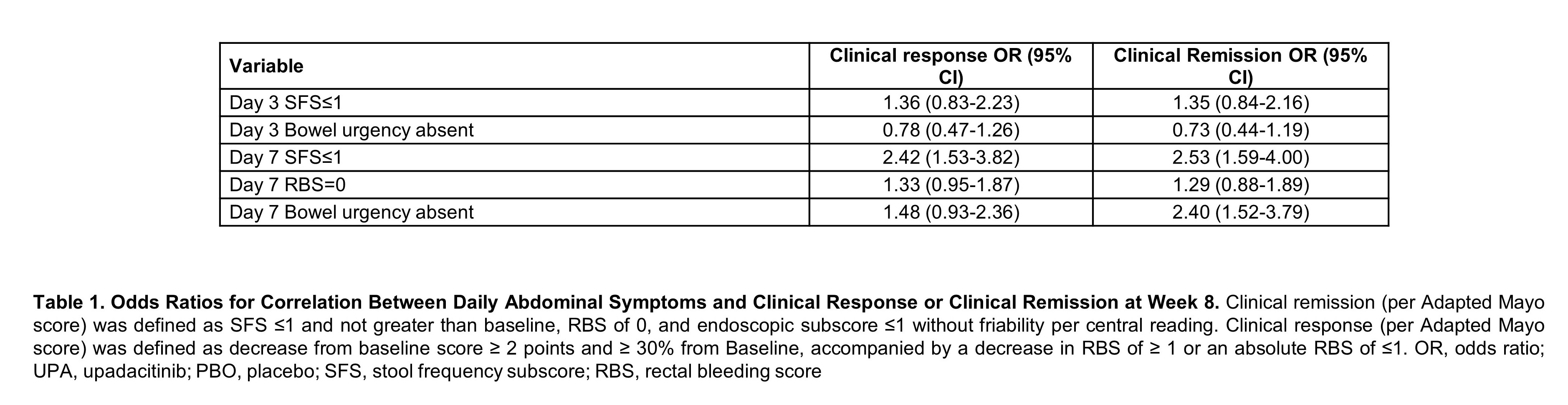
Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1
2022
ECCO'22 Virtual
Tuesday, 24 May 2022, 8:13 PM
Upper GI CD: What are the surgical options?
2019
8th S-ECCO IBD Masterclass
Wednesday, 5 June 2019, 9:01 PM
Upper GI pathology in IBD – when is it really IBD?
2020
5th H-ECCO IBD Masterclass
Tuesday, 23 June 2020, 5:40 PM
Use of Nutritional Therapy in IBD
2014
Talking Heads
Friday, 22 February 2019, 4:43 PM by ECCO Administrator
Friday, 13 January 2023, 12:23 PM by ECCO Administrator